Design criteria for stable Pt/C fuel cell catalysts
- PMID: 24605273
- PMCID: PMC3943646
- DOI: 10.3762/bjnano.5.5
Design criteria for stable Pt/C fuel cell catalysts
Abstract
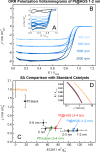
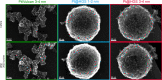
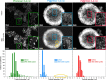
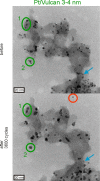
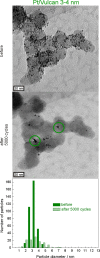
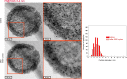
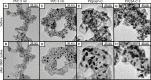
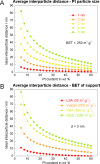
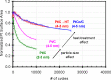
Platinum and Pt alloy nanoparticles supported on carbon are the state of the art electrocatalysts in proton exchange membrane fuel cells. To develop a better understanding on how material design can influence the degradation processes on the nanoscale, three specific Pt/C catalysts with different structural characteristics were investigated in depth: a conventional Pt/Vulcan catalyst with a particle size of 3-4 nm and two Pt@HGS catalysts with different particle size, 1-2 nm and 3-4 nm. Specifically, Pt@HGS corresponds to platinum nanoparticles incorporated and confined within the pore structure of the nanostructured carbon support, i.e., hollow graphitic spheres (HGS). All three materials are characterized by the same platinum loading, so that the differences in their performance can be correlated to the structural characteristics of each material. The comparison of the activity and stability behavior of the three catalysts, as obtained from thin film rotating disk electrode measurements and identical location electron microscopy, is also extended to commercial materials and used as a basis for a discussion of general fuel cell catalyst design principles. Namely, the effects of particle size, inter-particle distance, certain support characteristics and thermal treatment on the catalyst performance and in particular the catalyst stability are evaluated. Based on our results, a set of design criteria for more stable and active Pt/C and Pt-alloy/C materials is suggested.
Keywords: catalyst design criteria; degradation mechanisms; fuel cell catalyst; nanoparticles; stability.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
