Confinement dependence of electro-catalysts for hydrogen evolution from water splitting
- PMID: 24605286
- PMCID: PMC3943739
- DOI: 10.3762/bjnano.5.21
Confinement dependence of electro-catalysts for hydrogen evolution from water splitting
Abstract
Density functional theory is utilized to articulate a particular generic deconstruction of the electrode/electro-catalyst assembly for the cathode process during water splitting. A computational model was designed to determine how alloying elements control the fraction of H2 released during zirconium oxidation by water relative to the amount of hydrogen picked up by the corroding alloy. This model is utilized to determine the efficiencies of transition metals decorated with hydroxide interfaces in facilitating the electro-catalytic hydrogen evolution reaction. A computational strategy is developed to select an electro-catalyst for hydrogen evolution (HE), where the choice of a transition metal catalyst is guided by the confining environment. The latter may be recast into a nominal pressure experienced by the evolving H2 molecule. We arrived at a novel perspective on the uniqueness of oxide supported atomic Pt as a HE catalyst under ambient conditions.
Keywords: DFT; confinement; corrosion; electro-catalysis; hydrogen evolution.
Figures
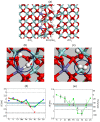
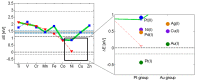

References
-
- Chlistunoff J. Final Technical Report - Advanced Chlor-Alkali Technology. Los Alamos, NM: Los Alamos National Laboratory; 2004.
-
- Lindgren M, Panas I. RSC Adv. 2013;3:21613–21619. doi: 10.1039/c3ra42941e. - DOI
-
- Lindgren M, Sundell G, Panas I, Hallstadius L, Thuvander M, Andrén H O. In: Comstock R J, editor. Zirconium in the Nuclear Industry: Seventeenth International Symposium; 2013 Feb 3-7; Hyderabad. West Conshohocken, PA: ASTM; p. tentatively accepted.
LinkOut - more resources
Full Text Sources
Other Literature Sources
