Adiponectin inhibits insulin function in primary trophoblasts by PPARα-mediated ceramide synthesis
- PMID: 24606127
- PMCID: PMC3968401
- DOI: 10.1210/me.2013-1401
Adiponectin inhibits insulin function in primary trophoblasts by PPARα-mediated ceramide synthesis
Abstract
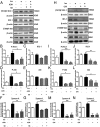
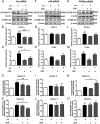
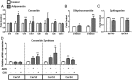
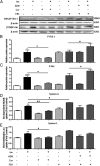
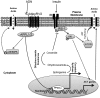
Maternal adiponectin (ADN) levels are inversely correlated with birth weight, and ADN infusion in pregnant mice down-regulates placental nutrient transporters and decreases fetal growth. In contrast to the insulin-sensitizing effects in adipose tissue and muscle, ADN inhibits insulin signaling in the placenta. However, the molecular mechanisms involved are unknown. We hypothesized that ADN inhibits insulin signaling and insulin-stimulated amino acid transport in primary human trophoblasts by peroxisome proliferator-activated receptor-α (PPARα)-mediated ceramide synthesis. Primary human term trophoblast cells were treated with ADN and/or insulin. ADN increased the phosphorylation of p38 MAPK and PPARα. ADN inhibited insulin signaling and insulin-stimulated amino acid transport. This effect was dependent on PPARα, because activation of PPARα with an agonist (GW7647) inhibited insulin signaling and function, whereas PPARα-small interfering RNA reversed the effects of ADN on the insulin response. ADN increased ceramide synthase expression and stimulated ceramide production. C2-ceramide inhibited insulin signaling and function, whereas inhibition of ceramide synthase (with Fumonisin B1) reversed the effects of ADN on insulin signaling and amino acid transport. These findings are consistent with the model that maternal ADN limits fetal growth mediated by activation of placental PPARα and ceramide synthesis, which inhibits placental insulin signaling and amino acid transport, resulting in reduced fetal nutrient availability.
Figures


References
-
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

