Hierarchy of RNA functional dynamics
- PMID: 24606137
- PMCID: PMC4048628
- DOI: 10.1146/annurev-biochem-060713-035524
Hierarchy of RNA functional dynamics
Abstract
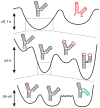
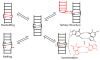
RNA dynamics play a fundamental role in many cellular functions. However, there is no general framework to describe these complex processes, which typically consist of many structural maneuvers that occur over timescales ranging from picoseconds to seconds. Here, we classify RNA dynamics into distinct modes representing transitions between basins on a hierarchical free-energy landscape. These transitions include large-scale secondary-structural transitions at >0.1-s timescales, base-pair/tertiary dynamics at microsecond-to-millisecond timescales, stacking dynamics at timescales ranging from nanoseconds to microseconds, and other "jittering" motions at timescales ranging from picoseconds to nanoseconds. We review various modes within these three different tiers, the different mechanisms by which they are used to regulate function, and how they can be coupled together to achieve greater functional complexity.
Keywords: RNA catalysis; RNA flexibility; molecular adaptation; regulatory RNA; riboswitches.
Conflict of interest statement
H.M.A. is an advisor to and holds an ownership interest in Nymirum Inc., which is an RNA-based drug discovery company. The research reported in this article was performed by the University of Michigan faculty and students and was funded by an NIH contract to H.M.A.
Figures



References
-
- Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE, Cech TR. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982;31:147–57. - PubMed
-
- Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35:849–57. - PubMed
-
- Voorhees RM, Ramakrishnan V. Structural basis of the translational elongation cycle. Annu Rev Biochem. 2013;82:203–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

