CERAMENT treatment of fracture defects (CERTiFy): protocol for a prospective, multicenter, randomized study investigating the use of CERAMENT™ BONE VOID FILLER in tibial plateau fractures
- PMID: 24606670
- PMCID: PMC3975294
- DOI: 10.1186/1745-6215-15-75
CERAMENT treatment of fracture defects (CERTiFy): protocol for a prospective, multicenter, randomized study investigating the use of CERAMENT™ BONE VOID FILLER in tibial plateau fractures
Abstract
Background: Bone graft substitutes are widely used for reconstruction of posttraumatic bone defects. However, their clinical significance in comparison to autologous bone grafting, the gold-standard in reconstruction of larger bone defects, still remains under debate. This prospective, randomized, controlled clinical study investigates the differences in pain, quality of life, and cost of care in the treatment of tibia plateau fractures-associated bone defects using either autologous bone grafting or bioresorbable hydroxyapatite/calcium sulphate cement (CERAMENT™|BONE VOID FILLER (CBVF)).
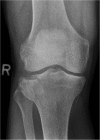
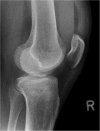
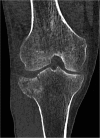
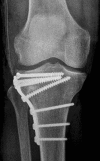
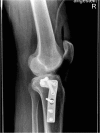
Methods/design: CERTiFy (CERament™ Treatment of Fracture defects) is a prospective, multicenter, controlled, randomized trial. We plan to enroll 136 patients with fresh traumatic depression fractures of the proximal tibia (types AO 41-B2 and AO 41-B3) in 13 participating centers in Germany. Patients will be randomized to receive either autologous iliac crest bone graft or CBVF after reduction and osteosynthesis of the fracture to reconstruct the subchondral bone defect and prevent the subsidence of the articular surface. The primary outcome is the SF-12 Physical Component Summary at week 26. The co-primary endpoint is the pain level 26 weeks after surgery measured by a visual analog scale. The SF-12 Mental Component Summary after 26 weeks and costs of care will serve as key secondary endpoints. The study is designed to show non-inferiority of the CBVF treatment to the autologous iliac crest bone graft with respect to the physical component of quality of life. The pain level at 26 weeks after surgery is expected to be lower in the CERAMENT bone void filler treatment group.
Discussion: CERTiFy is the first randomized multicenter clinical trial designed to compare quality of life, pain, and cost of care in the use of the CBVF and the autologous iliac crest bone graft in the treatment of tibia plateau fractures. The results are expected to influence future treatment recommendations.
Trial registration number: ClinicalTrials.gov: NCT01828905.
Figures
Similar articles
-
Autologous Iliac Bone Graft Compared with Biphasic Hydroxyapatite and Calcium Sulfate Cement for the Treatment of Bone Defects in Tibial Plateau Fractures: A Prospective, Randomized, Open-Label, Multicenter Study.J Bone Joint Surg Am. 2020 Feb 5;102(3):179-193. doi: 10.2106/JBJS.19.00680. J Bone Joint Surg Am. 2020. PMID: 31809394 Free PMC article. Clinical Trial.
-
Augmentation of tibial plateau fractures with an injectable bone substitute: CERAMENT™. Three year follow-up from a prospective study.BMC Musculoskelet Disord. 2015 May 13;16:115. doi: 10.1186/s12891-015-0574-6. BMC Musculoskelet Disord. 2015. PMID: 25968241 Free PMC article.
-
Use of bone graft substitutes in the management of tibial plateau fractures.Injury. 2013 Jan;44 Suppl 1:S86-94. doi: 10.1016/S0020-1383(13)70019-6. Injury. 2013. PMID: 23351879
-
Incidence of donor site morbidity following harvesting from iliac crest or RIA graft.Injury. 2014 Dec;45 Suppl 6:S116-20. doi: 10.1016/j.injury.2014.10.034. Epub 2014 Oct 27. Injury. 2014. PMID: 25457330 Review.
-
The use of bone-graft substitutes in large bone defects: any specific needs?Injury. 2011 Sep;42 Suppl 2:S56-63. doi: 10.1016/j.injury.2011.06.011. Epub 2011 Jul 12. Injury. 2011. PMID: 21752369 Review.
Cited by
-
Micro-Structural and Biomechanical Evaluation of Bioresorbable and Conventional Bone Cements for Augmentation of the Proximal Femoral Nail.J Clin Med. 2023 Nov 21;12(23):7202. doi: 10.3390/jcm12237202. J Clin Med. 2023. PMID: 38068254 Free PMC article.
-
Recent progress in the diagnosis and treatment of posterior tibial plateau fractures.Int J Clin Exp Med. 2015 Apr 15;8(4):5640-8. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26131147 Free PMC article.
-
Bone Tissue Engineering in the Treatment of Bone Defects.Pharmaceuticals (Basel). 2022 Jul 17;15(7):879. doi: 10.3390/ph15070879. Pharmaceuticals (Basel). 2022. PMID: 35890177 Free PMC article. Review.
-
Use of a biphasic cement bone substitute in the management of metaphyseal fractures.J Clin Orthop Trauma. 2019 Jul-Aug;10(4):789-791. doi: 10.1016/j.jcot.2018.08.014. Epub 2018 Aug 10. J Clin Orthop Trauma. 2019. PMID: 31316256 Free PMC article.
-
Autologous Iliac Bone Graft Compared with Biphasic Hydroxyapatite and Calcium Sulfate Cement for the Treatment of Bone Defects in Tibial Plateau Fractures: A Prospective, Randomized, Open-Label, Multicenter Study.J Bone Joint Surg Am. 2020 Feb 5;102(3):179-193. doi: 10.2106/JBJS.19.00680. J Bone Joint Surg Am. 2020. PMID: 31809394 Free PMC article. Clinical Trial.
References
-
- Tscherne H, Lobenhoffer P. Tibial plateau fractures. Management and expected results. Clin Orthop Relat Res. 1993;292:87–100. - PubMed
-
- Koval KJ, Helfet DL. Tibial plateau fractures: evaluation and treatment. J Am Acad Orthop Surg. 1995;3:86–94. - PubMed
-
- Lachiewicz PF, Funcik T. Factors influencing the results of open reduction and internal fixation of tibial plateau fractures. Clin Orthop Relat Res. 1990;259:210–215. - PubMed
-
- Segal D, Franchi AV, Campanile J. Iliac autograft for reconstruction of severely depressed fracture of a lateral tibial plateau. Brief note. J Bone Joint Surg Am. 1985;67:1270–1272. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

