Conformational flexibility of human casein kinase catalytic subunit explored by metadynamics
- PMID: 24606937
- PMCID: PMC4026775
- DOI: 10.1016/j.bpj.2014.01.031
Conformational flexibility of human casein kinase catalytic subunit explored by metadynamics
Abstract
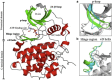
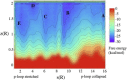
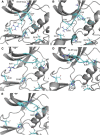
Casein kinase CK2 is an essential enzyme in higher organisms, catalyzing the transfer of the γ phosphate from ATP to serine and threonine residues on protein substrates. In a number of animal tumors, CK2 activity has been shown to escape normal cellular control, making it a potential target for cancer therapy. Several crystal structures of human CK2 have been published with different conformations for the CK2α catalytic subunit. This variability reflects a high flexibility for two regions of CK2α: the interdomain hinge region, and the glycine-rich loop (p-loop). Here, we present a computational study simulating the equilibrium between three conformations involving these regions. Simulations were performed using well-tempered metadynamics combined with a path collective variables approach. This provides a reference pathway describing the conformational changes being studied, based on analysis of free energy surfaces. The free energies of the three conformations were found to be close and the paths proposed had low activation barriers. Our results indicate that these conformations can exist in water. This information should be useful when designing inhibitors specific to one conformation.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Conformational dynamics of human protein kinase CK2α and its effect on function and inhibition.Proteins. 2018 Mar;86(3):344-353. doi: 10.1002/prot.25444. Epub 2017 Dec 25. Proteins. 2018. PMID: 29243286
-
Conformational plasticity of the catalytic subunit of protein kinase CK2 and its consequences for regulation and drug design.Biochim Biophys Acta. 2010 Mar;1804(3):484-92. doi: 10.1016/j.bbapap.2009.09.022. Epub 2009 Sep 28. Biochim Biophys Acta. 2010. PMID: 19796713 Review.
-
First inactive conformation of CK2 alpha, the catalytic subunit of protein kinase CK2.J Mol Biol. 2009 Mar 13;386(5):1212-21. doi: 10.1016/j.jmb.2009.01.033. Epub 2009 Jan 24. J Mol Biol. 2009. PMID: 19361447
-
Structure of the human protein kinase CK2 catalytic subunit CK2α' and interaction thermodynamics with the regulatory subunit CK2β.J Mol Biol. 2011 Mar 18;407(1):1-12. doi: 10.1016/j.jmb.2011.01.020. Epub 2011 Jan 15. J Mol Biol. 2011. PMID: 21241709
-
Protein kinase CK2 in health and disease: Protein kinase CK2: from structures to insights.Cell Mol Life Sci. 2009 Jun;66(11-12):1800-16. doi: 10.1007/s00018-009-9149-8. Cell Mol Life Sci. 2009. PMID: 19387553 Free PMC article. Review.
Cited by
-
Structural Hypervariability of the Two Human Protein Kinase CK2 Catalytic Subunit Paralogs Revealed by Complex Structures with a Flavonol- and a Thieno[2,3-d]pyrimidine-Based Inhibitor.Pharmaceuticals (Basel). 2017 Jan 11;10(1):9. doi: 10.3390/ph10010009. Pharmaceuticals (Basel). 2017. PMID: 28085026 Free PMC article.
-
Cancer selective cell death induction by a bivalent CK2 inhibitor targeting the ATP site and the allosteric αD pocket.iScience. 2024 Jan 12;27(2):108903. doi: 10.1016/j.isci.2024.108903. eCollection 2024 Feb 16. iScience. 2024. PMID: 38318383 Free PMC article.
-
2-Aminothiazole Derivatives as Selective Allosteric Modulators of the Protein Kinase CK2. 1. Identification of an Allosteric Binding Site.J Med Chem. 2019 Feb 28;62(4):1803-1816. doi: 10.1021/acs.jmedchem.8b01766. Epub 2019 Feb 18. J Med Chem. 2019. PMID: 30689953 Free PMC article.
-
Specific inhibition of CK2α from an anchor outside the active site.Chem Sci. 2016 Nov 1;7(11):6839-6845. doi: 10.1039/c6sc02335e. Epub 2016 Jul 12. Chem Sci. 2016. PMID: 28451126 Free PMC article.
-
Diacritic Binding of an Indenoindole Inhibitor by CK2α Paralogs Explored by a Reliable Path to Atomic Resolution CK2α' Structures.ACS Omega. 2019 Mar 19;4(3):5471-5478. doi: 10.1021/acsomega.8b03415. eCollection 2019 Mar 31. ACS Omega. 2019. PMID: 31559376 Free PMC article.
References
-
- Tawfic S., Yu S., Ahmed K. Protein kinase CK2 signal in neoplasia. Histol. Histopathol. 2001;16:573–582. - PubMed
-
- Papinutto E., Ranchio A., Battistutta R. Structural and functional analysis of the flexible regions of the catalytic α-subunit of protein kinase CK2. J. Struct. Biol. 2012;177:382–391. - PubMed
-
- Klopffleisch K., Issinger O.G., Niefind K. Low-density crystal packing of human protein kinase CK2 catalytic subunit in complex with resorufin or other ligands: a tool to study the unique hinge-region plasticity of the enzyme without packing bias. Acta Crystallogr. D Biol. Crystallogr. 2012;68:883–892. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

