Enhancement of tunability of MAPK cascade due to coexistence of processive and distributive phosphorylation mechanisms
- PMID: 24606945
- PMCID: PMC4026786
- DOI: 10.1016/j.bpj.2014.01.036
Enhancement of tunability of MAPK cascade due to coexistence of processive and distributive phosphorylation mechanisms
Abstract
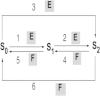
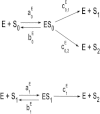
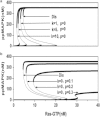
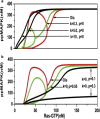
The processive phosphorylation mechanism becomes important when there is macromolecular crowding in the cytoplasm. Integrating the processive phosphorylation mechanism with the traditional distributive one, we propose a mixed dual-site phosphorylation (MDP) mechanism in a single-layer phosphorylation cycle. Further, we build a degree model by applying the MDP mechanism to a three-layer mitogen-activated protein kinase (MAPK) cascade. By bifurcation analysis, our study suggests that the crowded-environment-induced pseudoprocessive mechanism can qualitatively change the response of this biological network. By adjusting the degree of processivity in our model, we find that the MAPK cascade is able to switch between the ultrasensitivity, bistability, and oscillatory dynamical states. Sensitivity analysis shows that the theoretical results remain unchanged within a reasonably chosen variation of parameter perturbation. By scaling the reaction rates and also introducing new connections into the kinetic scheme, we further construct a proportion model of the MAPK cascade to validate our findings. Finally, it is illustrated that the spatial propagation of the activated MAPK signal can be improved (or attenuated) by increasing the degree of processivity of kinase (or phosphatase). Our research implies that the MDP mechanism makes the MAPK cascade become a flexible signal module, and the coexistence of processive and distributive phosphorylation mechanisms enhances the tunability of the MAPK cascade.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
The robustness of proofreading to crowding-induced pseudo-processivity in the MAPK pathway.Biophys J. 2014 Nov 18;107(10):2425-35. doi: 10.1016/j.bpj.2014.10.020. Biophys J. 2014. PMID: 25418311 Free PMC article.
-
Spatio-temporal correlations can drastically change the response of a MAPK pathway.Proc Natl Acad Sci U S A. 2010 Feb 9;107(6):2473-8. doi: 10.1073/pnas.0906885107. Epub 2010 Jan 25. Proc Natl Acad Sci U S A. 2010. PMID: 20133748 Free PMC article.
-
Processive phosphorylation of ERK MAP kinase in mammalian cells.Proc Natl Acad Sci U S A. 2011 Aug 2;108(31):12675-80. doi: 10.1073/pnas.1104030108. Epub 2011 Jul 18. Proc Natl Acad Sci U S A. 2011. PMID: 21768338 Free PMC article.
-
The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory.J Neurochem. 2001 Jan;76(1):1-10. doi: 10.1046/j.1471-4159.2001.00054.x. J Neurochem. 2001. PMID: 11145972 Review.
-
MAPK signaling in equations and embryos.Fly (Austin). 2009 Jan-Mar;3(1):62-7. doi: 10.4161/fly.3.1.7776. Epub 2009 Jan 6. Fly (Austin). 2009. PMID: 19182542 Free PMC article. Review.
Cited by
-
Interplay between Mdm2 and HIPK2 in the DNA damage response.J R Soc Interface. 2014 Jul 6;11(96):20140319. doi: 10.1098/rsif.2014.0319. J R Soc Interface. 2014. PMID: 24829283 Free PMC article.
-
Processivity in Bacterial Glycosyltransferases.ACS Chem Biol. 2020 Jan 17;15(1):3-16. doi: 10.1021/acschembio.9b00619. Epub 2019 Dec 11. ACS Chem Biol. 2020. PMID: 31750644 Free PMC article. Review.
-
Positive feedback induces switch between distributive and processive phosphorylation of Hog1.Nat Commun. 2023 Apr 29;14(1):2477. doi: 10.1038/s41467-023-37430-y. Nat Commun. 2023. PMID: 37120434 Free PMC article.
-
Kinase Inhibition Leads to Hormesis in a Dual Phosphorylation-Dephosphorylation Cycle.PLoS Comput Biol. 2016 Nov 29;12(11):e1005216. doi: 10.1371/journal.pcbi.1005216. eCollection 2016 Nov. PLoS Comput Biol. 2016. PMID: 27898662 Free PMC article.
References
-
- Manning G., Plowman G.D., Sudarsanam S. Evolution of protein kinase signaling from yeast to man. Trends Biochem. Sci. 2002;27:514–520. - PubMed
-
- Mellor J. Dynamic nucleosomes and gene transcription. Trends Genet. 2006;22:320–329. - PubMed
-
- Schofield C.J., Ratcliffe P.J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 2004;5:343–354. - PubMed
-
- Hershko A., Ciechanover A. The ubiquitin system. Annu. Rev. Biochem. 1998;67:425–479. - PubMed
-
- Salazar C., Höfer T. Multisite protein phosphorylation—from molecular mechanisms to kinetic models. FEBS J. 2009;276:3177–3198. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

