Distinct functional and pharmacological properties of Triheteromeric GluN1/GluN2A/GluN2B NMDA receptors
- PMID: 24607230
- PMCID: PMC3957490
- DOI: 10.1016/j.neuron.2014.01.035
Distinct functional and pharmacological properties of Triheteromeric GluN1/GluN2A/GluN2B NMDA receptors
Abstract
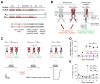
NMDA receptors are tetrameric ligand-gated ion channels comprised of GluN1, GluN2, and GluN3 subunits. Two different GluN2 subunits have been identified in most NMDA receptor-expressing cells, and the majority of native receptors are triheteromers containing two GluN1 and two different GluN2. In contrast to diheteromeric NMDA receptors, little is known about the function of triheteromers. We developed a method to provide selective cell-surface expression of recombinant GluN1/GluN2A/GluN2B triheteromers and compared properties of these receptors with those of GluN1/GluN2A and GluN1/GluN2B diheteromers. We show that glutamate deactivation of triheteromers is distinct from those of GluN1/GluN2A and GluN1/GluN2B and reveal modulation of triheteromers by subunit-selective antagonists ifenprodil, CP-101,606, TCN-201, and extracellular Zn(2+). Furthermore, kinetic measurements suggest variation in the ifenprodil binding site of triheteromers compared to GluN1/GluN2B diheteromers. This work provides insight into the distinct properties of GluN1/GluN2A/GluN2B triheteromers, which are presumably the most abundant NMDA receptors in the adult forebrain.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




References
-
- Arai R, Wriggers W, Nishikawa Y, Nagamune T, Fujisawa T. Conformations of variably linked chimeric proteins evaluated by synchrotron X-ray small-angle scattering. Proteins. 2004;57:829–838. - PubMed
-
- Bard L, Groc L. Glutamate receptor dynamics and protein interaction: lessons from the NMDA receptor. Mol Cell Neurosci. 2011;48:298–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

