Metastatic stem cells: sources, niches, and vital pathways
- PMID: 24607405
- PMCID: PMC3998185
- DOI: 10.1016/j.stem.2014.02.002
Metastatic stem cells: sources, niches, and vital pathways
Abstract
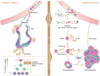
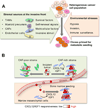
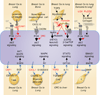
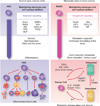
Metastasis is powered by disseminated cancer cells that re-create a full-fledged tumor in unwelcoming tissues, away from the primary site. How cancer cells moving from a tumor into the circulation manage to infiltrate distant organs and initiate metastatic growth is of interest to cancer biologists and clinical oncologists alike. Recent findings have started to define the sources, phenotypic properties, hosting niches, and signaling pathways that support the survival, self-renewal, dormancy, and reactivation of cancer cells that initiate metastasis: metastatic stem cells. By dissecting the biology of this process, vulnerabilities are being exposed that could be exploited to prevent metastasis.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



References
-
- Aguirre-Ghiso JA, Ossowski L, Rosenbaum SK. Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Res. 2004;64:7336–7345. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

