Wounding the cornea to learn how it heals
- PMID: 24607489
- PMCID: PMC4072315
- DOI: 10.1016/j.exer.2014.02.007
Wounding the cornea to learn how it heals
Abstract
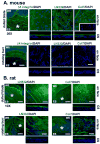
Corneal wound healing studies have a long history and rich literature that describes the data obtained over the past 70 years using many different species of animals and methods of injury. These studies have lead to reduced suffering and provided clues to treatments that are now helping patients live more productive lives. In spite of the progress made, further research is required since blindness and reduced quality of life due to corneal scarring still happens. The purpose of this review is to summarize what is known about different types of wound and animal models used to study corneal wound healing. The subject of corneal wound healing is broad and includes chemical and mechanical wound models. This review focuses on mechanical injury models involving debridement and keratectomy wounds to reflect the authors' expertise.
Keywords: animal models; cornea; debridement; keratectomy; mouse strain; wound healing.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


References
-
- Acheampong AA, Shackleton M, Tang-Liu DD, Ding S, Stern ME, Decker R. Distribution of cyclosporin A in ocular tissues after topical administration to albino rabbits and beagle dogs. Current eye research. 1999;18:91–103. - PubMed
-
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318:533–538. - PubMed
-
- Amescua G, Collings F, Sidani A, Bonfield TL, Rodriguez JP, Galor A, Medina C, Yang X, Perez VL. Effect of CXCL-1/KC production in high risk vascularized corneal allografts on T cell recruitment and graft rejection. Transplantation. 2008;85:615–625. - PubMed
-
- Anderson RA. Actin filaments in normal and migrating corneal epithelial cells. Investigative ophthalmology & visual science. 1977;16:161–166. - PubMed
-
- Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Investigative ophthalmology & visual science. 1995;36:614–621. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 EY020886/EY/NEI NIH HHS/United States
- EY021784/EY/NEI NIH HHS/United States
- EY023106/EY/NEI NIH HHS/United States
- EY008512/EY/NEI NIH HHS/United States
- EY005665/EY/NEI NIH HHS/United States
- R01 EY006000/EY/NEI NIH HHS/United States
- EYO6000S/PHS HHS/United States
- R01 EY021784/EY/NEI NIH HHS/United States
- R21 EY023106/EY/NEI NIH HHS/United States
- R01 EY008512/EY/NEI NIH HHS/United States
- P30 EY003790/EY/NEI NIH HHS/United States
- R01 EY005665/EY/NEI NIH HHS/United States
- EY06000/EY/NEI NIH HHS/United States
- EY003790/EY/NEI NIH HHS/United States
- EY020886/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

