Mild cognitive impairment and asymptomatic Alzheimer disease subjects: equivalent β-amyloid and tau loads with divergent cognitive outcomes
- PMID: 24607960
- PMCID: PMC4062187
- DOI: 10.1097/NEN.0000000000000052
Mild cognitive impairment and asymptomatic Alzheimer disease subjects: equivalent β-amyloid and tau loads with divergent cognitive outcomes
Abstract
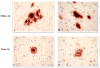
Older adults with intact cognition before death and substantial Alzheimer disease (AD) lesions at autopsy have been termed "asymptomatic AD subjects" (ASYMAD). We previously reported hypertrophy of neuronal cell bodies, nuclei, and nucleoli in the CA1 of the hippocampus (CA1), anterior cingulate gyrus, posterior cingulate gyrus, and primary visual cortex of ASYMAD versus age-matched Control and mild cognitive impairment (MCI) subjects. However, it was unclear whether the neuronal hypertrophy could be attributed to differences in the severity of AD pathology. Here, we performed quantitative analyses of the severity of β-amyloid (Aβ) and phosphorylated tau (tau) loads in the brains of ASYMAD, Control, MCI, and AD subjects (n = 15 per group) from the Baltimore Longitudinal Study of Aging. Tissue sections from CA1, anterior cingulate gyrus, posterior cingulate gyrus, and primary visual cortex were immunostained for Aβ and tau; the respective loads were assessed using unbiased stereology by measuring the fractional areas of immunoreactivity for each protein in each region. The ASYMAD and MCI groups did not differ in Aβ and tau loads. These data confirm that ASYMAD and MCI subjects have comparable loads of insoluble Aβ and tau in regions vulnerable to AD pathology despite divergent cognitive outcomes. These findings imply that cognitive impairment in AD may be caused or modulated by factors other than insoluble forms of Aβ and tau.
Figures





References
-
- Tomlinson BE, Blessed G, Roth M. Observations on the brains of nondemented old people. J Neurol Sci. 1968;7:331–56. - PubMed
-
- Katzman R, Terry R, DeTeresa R, et al. Clinical, pathological, and neurochemical changes in dementia: A subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–44. - PubMed
-
- Crystal H, Dickson D, Fuld P, et al. Clinicopathologic studies in dementia: Nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology. 1988;38:1682–87. - PubMed
-
- Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–95. - PubMed
-
- Troncoso JC, Martin LJ, Dal Forno G, et al. Neuropathology in controls and demented subjects from the Baltimore Longitudinal Study of Aging. Neurobiol Aging. 1996;17:365–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

