Anion exchange HPLC isolation of high-density lipoprotein (HDL) and on-line estimation of proinflammatory HDL
- PMID: 24609013
- PMCID: PMC3946658
- DOI: 10.1371/journal.pone.0091089
Anion exchange HPLC isolation of high-density lipoprotein (HDL) and on-line estimation of proinflammatory HDL
Abstract
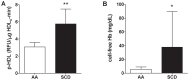
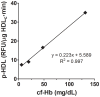
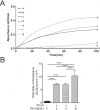
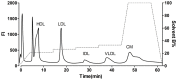
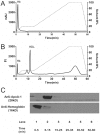
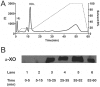
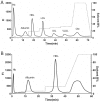
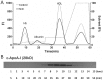
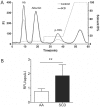
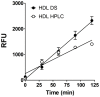
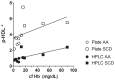
Proinflammatory high-density lipoprotein (p-HDL) is a biomarker of cardiovascular disease. Sickle cell disease (SCD) is characterized by chronic states of oxidative stress that many consider to play a role in forming p-HDL. To measure p-HDL, apolipoprotein (apo) B containing lipoproteins are precipitated. Supernatant HDL is incubated with an oxidant/LDL or an oxidant alone and rates of HDL oxidation monitored with dichlorofluorescein (DCFH). Although apoB precipitation is convenient for isolating HDL, the resulting supernatant matrix likely influences HDL oxidation. To determine effects of supernatants on p-HDL measurements we purified HDL from plasma from SCD subjects by anion exchange (AE) chromatography, determined its rate of oxidation relative to supernatant HDL. SCD decreased total cholesterol but not triglycerides or HDL and increased cell-free (cf) hemoglobin (Hb) and xanthine oxidase (XO). HDL isolated by AE-HPLC had lower p-HDL levels than HDL in supernatants after apoB precipitation. XO+xanthine (X) and cf Hb accelerated purified HDL oxidation. Although the plate and AE-HPLC assays both showed p-HDL directly correlated with cf-Hb in SCD plasma, the plate assay yielded p-HDL data that was influenced more by cf-Hb than AE-HPLC generated p-HDL data. The AE-HPLC p-HDL assay reduces the influence of the supernatants and shows that SCD increases p-HDL.
Conflict of interest statement
Figures
References
-
- Assmann G, von Eckardstein A, Funke H (1993) High density lipoproteins, reverse transport of cholesterol, and coronary artery disease. Insights from mutations. Circulation 87: III28–34. - PubMed
-
- Navab M, Fogelman AM, Berliner JA, Territo MC, Demer LL, et al. (1995) Pathogenesis of atherosclerosis. Am J Cardiol 76: 18C–23C. - PubMed
-
- Gharavi NM, Gargalovic PS, Chang I, Araujo JA, Clark MJ, et al. (2007) High-density lipoprotein modulates oxidized phospholipid signaling in human endothelial cells from proinflammatory to anti-inflammatory. Arterioscler Thromb Vasc Biol 27: 1346–1353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

