Cost-effectiveness analysis of universal influenza vaccination with quadrivalent inactivated vaccine in the United States
- PMID: 24609063
- PMCID: PMC4896600
- DOI: 10.4161/hv.28221
Cost-effectiveness analysis of universal influenza vaccination with quadrivalent inactivated vaccine in the United States
Abstract
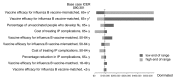
To address influenza B lineage mismatch and co-circulation, several quadrivalent inactivated influenza vaccines (IIV4s) containing two type A strains and both type B lineages have recently been approved in the United States. Currently available trivalent inactivated vaccines (IIV3s) or trivalent live attenuated influenza vaccines (LAIV3s) comprise two influenza A strains and one of the two influenza B lineages that have co-circulated in the United States since 2001. The objective of this analysis was to evaluate the cost-effectiveness of a policy of universal vaccination with IIV4 vs. IIV3/LAIV3 during 1 year in the United States. On average per influenza season, IIV4 was predicted to result in 30,251 fewer influenza cases, 3512 fewer hospitalizations, 722 fewer deaths, 4812 fewer life-years lost, and 3596 fewer quality-adjusted life-years (QALYs) lost vs. IIV3/LAIV3. Using the Fluarix Quadrivalent(TM) (GlaxoSmithKline) prices and the weighted average IIV3/LAIV3 prices, the model predicts that the vaccination program costs would increase by $452.2 million, while direct medical and indirect costs would decrease by $111.6 million and $218.7 million, respectively, with IIV4. The incremental cost-effectiveness ratio (ICER) comparing IIV4 to IIV3/LAIV3 is predicted to be $90,301/QALY gained. Deterministic sensitivity analyses found that influenza B vaccine-matched and mismatched efficacies among adults aged ≥65 years had the greatest impact on the ICER. Probabilistic sensitivity analysis showed that the cost per QALY remained below $100,000 for 61% of iterations. In conclusion, vaccination with IIV4 in the US is predicted to reduce morbidity and mortality. This strategy is also predicted to be cost-effective vs. IIV3/LAIV3 at conventional willingness-to-pay thresholds.
Keywords: Markov model; United States; cost-effectiveness; influenza; quadrivalent inactivated vaccine.
Figures
References
-
- Centers for Disease Control and Prevention (CDC). Seasonal Influenza (Flu): Questions & Answers. Available from: http://www.cdc.gov/flu/about/qa/preventingflu.htm
-
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. . The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086 - 96; http://dx.doi.org/ 10.1016/j.vaccine.2007.03.046; PMID: 17544181 - DOI - PubMed
-
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, et al. , Centers for Disease Control and Prevention (CDC). . Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:RR-8 1 - 62; PMID: 20689501 - PubMed
-
- Centers for Disease Control and Prevention (CDC). . Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)--United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep 2012; 61:613 - 8; PMID: 22895385 - PubMed
-
- Centers for Disease Control and Prevention (CDC). Flu Activity & Surveillance Reports.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical