In vivo detection of peripherin-specific autoreactive B cells during type 1 diabetes pathogenesis
- PMID: 24610011
- PMCID: PMC3994320
- DOI: 10.4049/jimmunol.1301053
In vivo detection of peripherin-specific autoreactive B cells during type 1 diabetes pathogenesis
Abstract
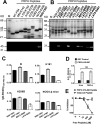
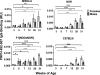
Autoreactive B cells are essential for the pathogenesis of type 1 diabetes. The genesis and dynamics of autoreactive B cells remain unknown. In this study, we analyzed the immune response in the NOD mouse model to the neuronal protein peripherin (PRPH), a target Ag of islet-infiltrating B cells. PRPH autoreactive B cells recognized a single linear epitope of this protein, in contrast to the multiple epitope recognition commonly observed during autoreactive B cell responses. Autoantibodies to this epitope were also detected in the disease-resistant NOR and C57BL/6 strains. To specifically detect the accumulation of these B cells, we developed a novel approach, octameric peptide display, to follow the dynamics and localization of anti-PRPH B cells during disease progression. Before extended insulitis was established, anti-PRPH B cells preferentially accumulated in the peritoneum. Anti-PRPH B cells were likewise detected in C57BL/6 mice, albeit at lower frequencies. As disease unfolded in NOD mice, anti-PRPH B cells invaded the islets and increased in number at the peritoneum of diabetic but not prediabetic mice. Isotype-switched B cells were only detected in the peritoneum. Anti-PRPH B cells represent a heterogeneous population composed of both B1 and B2 subsets. In the spleen, anti-PRPH B cell were predominantly in the follicular subset. Therefore, anti-PRPH B cells represent a heterogeneous population that is generated early in life but proliferates as diabetes is established. These findings on the temporal and spatial progression of autoreactive B cells should be relevant for our understanding of B cell function in diabetes pathogenesis.
Figures




Similar articles
-
Peripherin is a relevant neuroendocrine autoantigen recognized by islet-infiltrating B lymphocytes.J Immunol. 2007 May 15;178(10):6533-9. doi: 10.4049/jimmunol.178.10.6533. J Immunol. 2007. PMID: 17475883
-
Peripherin-reactive antibodies in mouse, rabbit, and human blood.J Proteome Res. 2010 Mar 5;9(3):1203-8. doi: 10.1021/pr900492y. J Proteome Res. 2010. PMID: 20113007 Free PMC article.
-
B-cells are required for the initiation of insulitis and sialitis in nonobese diabetic mice.Diabetes. 1997 Jun;46(6):941-6. doi: 10.2337/diab.46.6.941. Diabetes. 1997. PMID: 9166663
-
B cells and type 1 diabetes ...in mice and men.Immunol Lett. 2014 Aug;160(2):128-32. doi: 10.1016/j.imlet.2014.01.010. Epub 2014 Jan 25. Immunol Lett. 2014. PMID: 24472603 Free PMC article. Review.
-
B cells in the spotlight: innocent bystanders or major players in the pathogenesis of type 1 diabetes.Trends Endocrinol Metab. 2006 May-Jun;17(4):128-35. doi: 10.1016/j.tem.2006.03.006. Epub 2006 Apr 3. Trends Endocrinol Metab. 2006. PMID: 16580840 Review.
Cited by
-
Dysregulation of circulating follicular helper T cells in type 2 diabetic patients with diabetic retinopathy.Immunol Res. 2021 Apr;69(2):153-161. doi: 10.1007/s12026-021-09182-8. Epub 2021 Feb 24. Immunol Res. 2021. PMID: 33625683
-
Two to Tango: Dialogue between Adaptive and Innate Immunity in Type 1 Diabetes.J Diabetes Res. 2020 Jul 30;2020:4106518. doi: 10.1155/2020/4106518. eCollection 2020. J Diabetes Res. 2020. PMID: 32802890 Free PMC article. Review.
-
B-lymphocytes expressing an Ig specificity recognizing the pancreatic ß-cell autoantigen peripherin are potent contributors to type 1 diabetes development in NOD mice.Diabetes. 2016 Jul;65(7):1977-1987. doi: 10.2337/db15-1606. Epub 2016 Mar 9. Diabetes. 2016. PMID: 26961115 Free PMC article.
-
New Players in the Field of T1D: Anti-Peripherin B Lymphocytes.Diabetes. 2016 Jul;65(7):1794-6. doi: 10.2337/dbi16-0017. Diabetes. 2016. PMID: 27329956 Free PMC article. No abstract available.
-
Understanding and measuring human B-cell tolerance and its breakdown in autoimmune disease.Immunol Rev. 2019 Nov;292(1):76-89. doi: 10.1111/imr.12820. Epub 2019 Nov 22. Immunol Rev. 2019. PMID: 31755562 Free PMC article. Review.
References
-
- Favas C, Isenberg DA. B-cell-depletion therapy in SLE - what are the current prospects for its acceptance? Nat. Rev. Rheumatol. 2009;5:711–716. - PubMed
-
- Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat. Rev. Immunol. 2010;10:501–513. - PubMed
-
- Serreze DV, Chapman HD, Varnum DS, Hanson MS, Reifsnyder PC, Richard SD, Fleming SA, Leiter EH, Shultz LD. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu null mice. J. Exp. Med. 1996;184:2049–2053. - PMC - PubMed
-
- Akashi T, Nagafuchi S, Anzai K, Kondo S, Kitamura D, Wakana S, Ono J, Kikuchi M, Niho Y, Watanabe T. Direct evidence for the contribution of B cells to the progression of insulitis and the development of diabetes in non-obese diabetic mice. Int. Immunol. 1997;9:1159–1164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

