B cells in T follicular helper cell development and function: separable roles in delivery of ICOS ligand and antigen
- PMID: 24610013
- PMCID: PMC3991608
- DOI: 10.4049/jimmunol.1302617
B cells in T follicular helper cell development and function: separable roles in delivery of ICOS ligand and antigen
Abstract
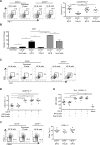
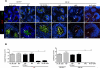
B cells are required for follicular Th (Tfh) cell development, as is the ICOS ligand (ICOS-L); however, the separable contributions of Ag and ICOS-L delivery by cognate B cells to Tfh cell development and function are unknown. We find that Tfh cell and germinal center differentiation are dependent on cognate B cell display of ICOS-L, but only when Ag presentation by the latter is limiting, with the requirement for B cell expression of ICOS-L overcome by robust Ag delivery. These findings demonstrate that Ag-specific B cells provide different, yet compensatory, signals for Tfh cell differentiation, while reconciling conflicting data indicating a requirement for ICOS-L expression on cognate B cells for Tfh cell development with those demonstrating that the latter requirement could be bypassed in lieu of that tendered by noncognate B cells. Our findings clarify the separable roles of delivery of Ag and ICOS-L by cognate B cells for Tfh cell maturation and function, and have implications for using therapeutic ICOS blockade in settings of abundantly available Ag, such as in systemic autoimmunity.
Figures






References
-
- King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. - PubMed
-
- Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

