Traumatic Brain Injury Increases Cortical Glutamate Network Activity by Compromising GABAergic Control
- PMID: 24610117
- PMCID: PMC4494035
- DOI: 10.1093/cercor/bhu041
Traumatic Brain Injury Increases Cortical Glutamate Network Activity by Compromising GABAergic Control
Abstract
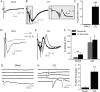
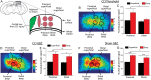
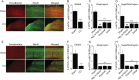
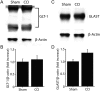
Traumatic brain injury (TBI) is a major risk factor for developing pharmaco-resistant epilepsy. Although disruptions in brain circuitry are associated with TBI, the precise mechanisms by which brain injury leads to epileptiform network activity is unknown. Using controlled cortical impact (CCI) as a model of TBI, we examined how cortical excitability and glutamatergic signaling was altered following injury. We optically mapped cortical glutamate signaling using FRET-based glutamate biosensors, while simultaneously recording cortical field potentials in acute brain slices 2-4 weeks following CCI. Cortical electrical stimulation evoked polyphasic, epileptiform field potentials and disrupted the input-output relationship in deep layers of CCI-injured cortex. High-speed glutamate biosensor imaging showed that glutamate signaling was significantly increased in the injured cortex. Elevated glutamate responses correlated with epileptiform activity, were highest directly adjacent to the injury, and spread via deep cortical layers. Immunoreactivity for markers of GABAergic interneurons were significantly decreased throughout CCI cortex. Lastly, spontaneous inhibitory postsynaptic current frequency decreased and spontaneous excitatory postsynaptic current increased after CCI injury. Our results suggest that specific cortical neuronal microcircuits may initiate and facilitate the spread of epileptiform activity following TBI. Increased glutamatergic signaling due to loss of GABAergic control may provide a mechanism by which TBI can give rise to post-traumatic epilepsy.
Keywords: cortical hyperexcitability; epilepsy; glutamate signaling; network reorganization; traumatic brain injury.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures





References
-
- Anderson CM, Swanson RA. 2000. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 32:1–14. - PubMed
-
- Annegers JF, Hauser WA, Coan SP, Rocca WA. 1998. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 338:20–24. - PubMed
-
- Asikainen I, Kaste M, Sarna S. 1999. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia. 40:584–589. - PubMed
-
- Bolkvadze T, Pitkanen A. 2012. Development of post-traumatic epilepsy after controlled cortical impact and lateral fluid-percussion-induced brain injury in the mouse. J Neurotrauma. 29:789–812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

