A novel ESX-1 locus reveals that surface-associated ESX-1 substrates mediate virulence in Mycobacterium marinum
- PMID: 24610712
- PMCID: PMC4011007
- DOI: 10.1128/JB.01502-14
A novel ESX-1 locus reveals that surface-associated ESX-1 substrates mediate virulence in Mycobacterium marinum
Abstract
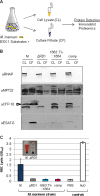
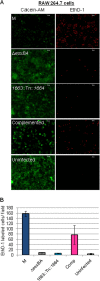
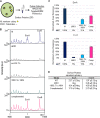
EsxA (ESAT-6) and EsxB (CFP-10) are virulence factors exported by the ESX-1 system in mycobacterial pathogens. In Mycobacterium marinum, an established model for ESX-1 secretion in Mycobacterium tuberculosis, genes required for ESX-1 export reside at the extended region of difference 1 (RD1) locus. In this study, a novel locus required for ESX-1 export in M. marinum was identified outside the RD1 locus. An M. marinum strain bearing a transposon-insertion between the MMAR_1663 and MMAR_1664 genes exhibited smooth-colony morphology, was deficient for ESX-1 export, was nonhemolytic, and was attenuated for virulence. Genetic complementation revealed a restoration of colony morphology and a partial restoration of virulence in cell culture models. Yet hemolysis and the export of ESX-1 substrates into the bacteriological medium in vitro as measured by both immunoblotting and quantitative proteomics were not restored. We show that genetic complementation of the transposon insertion strain partially restored the translocation of EsxA and EsxB to the mycobacterial cell surface. Our findings indicate that the export of EsxA and EsxB to the cell surface, rather than secretion into the bacteriological medium, correlates with virulence in M. marinum. Together, these findings not only expand the known genetic loci required for ESX-1 secretion in M. marinum but also provide an explanation for the observed disparity between in vitro ESX-1 export and virulence.
Figures


References
-
- Hsu T, Hingley-Wilson SM, Chen B, Chen M, Dai AZ, Morin PM, Marks CB, Padiyar J, Goulding C, Gingery M, Eisenberg D, Russell RG, Derrick SC, Collins FM, Morris SL, King CH, Jacobs WR., Jr 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. U. S. A. 100:12420–12425. 10.1073/pnas.1635213100 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

