Decreased cystathionine-γ-lyase (CSE) activity in livers of type 1 diabetic rats and peripheral blood mononuclear cells (PBMC) of type 1 diabetic patients
- PMID: 24610811
- PMCID: PMC4002085
- DOI: 10.1074/jbc.M113.524645
Decreased cystathionine-γ-lyase (CSE) activity in livers of type 1 diabetic rats and peripheral blood mononuclear cells (PBMC) of type 1 diabetic patients
Abstract
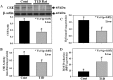
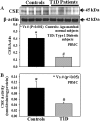
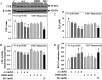
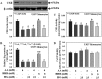
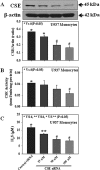
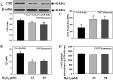
The liver plays a major role in the formation of H2S, a novel signaling molecule. Diabetes is associated with lower blood levels of H2S. This study investigated the activities of cystathionine-γ-lyase (CSE, the enzyme that catalyzes H2S formation) in livers of type 1 diabetic (T1D) animals and in peripheral blood mononuclear cells (PBMC) isolated from T1D patients. T1D is associated with both hyperketonemia (acetoacetate and β-hydroxybutyrate) and hyperglycemia. This study also examined the role of hyperglycemia and hyperketonemia per se in decreased CSE activity using U937 monocytes and PBMC isolated from healthy subjects. Livers from streptozotocin-treated T1D rats demonstrated a significantly higher reactive oxygen species production, lower CSE protein expression and activity, and lower H2S formation compared with those of controls. Studies with T1D patients showed a decrease in CSE protein expression and activity in PBMC compared with those of age-matched normal subjects. Cell culture studies demonstrated that high glucose (25 mm) and/or acetoacetate (4 mm) increased reactive oxygen species, decreased CSE mRNA expression, protein expression, and enzymatic activity, and reduced H2S levels; however, β-hydroxybutyrate treatment had no effect. A similar effect, which was also observed in PBMC treated with high glucose alone or along with acetoacetate, was prevented by vitamin D supplementation. Studies with CSE siRNA provide evidence for a relationship between impaired CSE expression and reduced H2S levels. This study demonstrates for the first time that both hyperglycemia and hyperketonemia mediate a reduction in CSE expression and activity, which can contribute to the impaired H2S signaling associated with diabetes.
Keywords: Acetoacetate; Diabetes; Glucose; Hydrogen Sulfide; Ketone Bodies.
Figures



References
-
- Elsey D. J., Fowkes R. C., Baxter G. F. (2010) Regulation of cardiovascular cell function by hydrogen sulfide (H2S). Cell Biochem. Funct. 28, 95–106 - PubMed
-
- Liu Y. H., Lu M., Hu L. F., Wong P. T., Webb G. D., Bian J. S. (2012) Hydrogen sulfide in the mammalian cardiovascular system. Antioxid. Redox Signal.. 17, 141–185 - PubMed
-
- Manna P., Jain S. K. (2013) l-Cysteine and hydrogen sulfide increase PIP3 and AMPK/PPARγ expression and decrease ROS and vascular inflammation markers in high glucose-treated human U937 monocytes. J. Cell Biochem. 114, 2334–2345 - PubMed
-
- Manna P., Jain S. K. (2011) Hydrogen sulfide and l-cysteine increase phosphatidylinositol 3,4,5-trisphosphate (PIP3) and glucose utilization by inhibiting phosphatase and tensin homolog (PTEN) protein and activating phosphoinositide 3-kinase (PI3K)/serine/threonine protein kinase (AKT)/protein kinase Cζ/λ (PKCζ/λ) in 3T3l1 adipocytes. J. Biol. Chem. 286, 39848–39859 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

