Risk of newly detected infections and cervical abnormalities in women seropositive for naturally acquired human papillomavirus type 16/18 antibodies: analysis of the control arm of PATRICIA
- PMID: 24610876
- PMCID: PMC4111909
- DOI: 10.1093/infdis/jiu139
Risk of newly detected infections and cervical abnormalities in women seropositive for naturally acquired human papillomavirus type 16/18 antibodies: analysis of the control arm of PATRICIA
Abstract
Background: We examined risk of newly detected human papillomavirus (HPV) infection and cervical abnormalities in relation to HPV type 16/18 antibody levels at enrollment in PATRICIA (Papilloma Trial Against Cancer in Young Adults; NCT00122681).
Methods: Using Poisson regression, we compared risk of newly detected infection and cervical abnormalities associated with HPV-16/18 between seronegative vs seropositive women (15-25 years) in the control arm (DNA negative at baseline for the corresponding HPV type [HPV-16: n = 8193; HPV-18: n = 8463]).
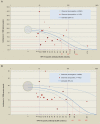
Results: High titers of naturally acquired HPV-16 antibodies and/or linear trend for increasing antibody levels were significantly associated with lower risk of incident and persistent infection, atypical squamous cells of undetermined significance or greater (ASCUS+), and cervical intraepithelial neoplasia grades 1/2 or greater (CIN1+, CIN2+). For HPV-18, although seropositivity was associated with lower risk of ASCUS+ and CIN1+, no association between naturally acquired antibodies and infection was demonstrated. Naturally acquired HPV-16 antibody levels of 371 (95% confidence interval [CI], 242-794), 204 (95% CI, 129-480), and 480 (95% CI, 250-5756) EU/mL were associated with 90% reduction of incident infection, 6-month persistent infection, and ASCUS+, respectively.
Conclusions: Naturally acquired antibodies to HPV-16, and to a lesser extent HPV-18, are associated with some reduced risk of subsequent infection and cervical abnormalities associated with the same HPV type.
Keywords: HPV; cervical abnormality; infection; naturally acquired antibodies; risk reduction.
© The Author 2014. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Figures




Comment in
-
Naturally acquired immunity against human papillomavirus (HPV): why it matters in the HPV vaccine era.J Infect Dis. 2014 Aug 15;210(4):507-9. doi: 10.1093/infdis/jiu143. Epub 2014 Mar 8. J Infect Dis. 2014. PMID: 24610878 No abstract available.
References
-
- de Sanjosé S, Quint WGV, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional survey. Lancet Oncol. 2010;11:1048–56. - PubMed
-
- The GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374:1975–85. - PubMed
-
- Paavonen J, Naud P, Salmerón J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. - PubMed
-
- Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43. - PubMed
-
- The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1916–27. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

