Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy
- PMID: 24610926
- PMCID: PMC4033365
- DOI: 10.1681/ASN.2013040430
Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy
Abstract
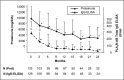
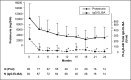
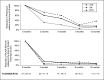
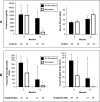
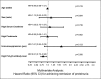
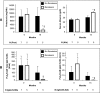
Membranous nephropathy (MN) is the most common cause of nephrotic syndrome in adults, with an uncertain clinical outcome. The characterization of the phospholipase A2 receptor (PLA2R) as the major target antigen in primary MN and the detection of circulating autoantibodies in these patients is a major advance in understanding this disease. To test whether PLA2R antibody levels reflect disease activity or clinical outcome, we performed a prospective multicenter study of 133 adult patients with primary MN and detectable serum PLA2R antibodies who had not received immunosuppressive therapy. Patients were followed ≤24 months. PLA2R antibody levels associated with clinical disease activity (proteinuria) in patients with immunosuppressive therapy (n=101) or supportive care (n=32). Within 3 months, immunosuppressive therapy led to a sustained 81% reduction in PLA2R antibody levels paralleled by a 39% reduction in proteinuria. Patients who experienced remission of proteinuria after 12 months had significantly lower PLA2R antibody levels at the time of study inclusion compared with patients with no remission. Patients with high PLA2R antibody levels achieved remission of proteinuria significantly later than patients with low PLA2R antibody levels. PLA2R antibody levels fell over time in patients with spontaneous remission but remained elevated in patients who did not show a reduction in proteinuria. Multivariable Cox regression analysis confirmed PLA2R antibody level as an independent risk factor for not achieving remission of proteinuria. We conclude that a decrease in PLA2R antibody level is associated with a decrease of proteinuria in patients with primary MN.
Copyright © 2014 by the American Society of Nephrology.
Figures

Comment in
-
Phospholipase A2 receptor antibodies in membranous nephropathy: unresolved issues.J Am Soc Nephrol. 2014 Jun;25(6):1137-9. doi: 10.1681/ASN.2014010091. Epub 2014 Mar 7. J Am Soc Nephrol. 2014. PMID: 24610931 Free PMC article. No abstract available.
References
-
- Schieppati A, Mosconi L, Perna A, Mecca G, Bertani T, Garattini S, Remuzzi G: Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med 329: 85–89, 1993 - PubMed
-
- Polanco N, Gutiérrez E, Covarsí A, Ariza F, Carreño A, Vigil A, Baltar J, Fernández-Fresnedo G, Martín C, Pons S, Lorenzo D, Bernis C, Arrizabalaga P, Fernández-Juárez G, Barrio V, Sierra M, Castellanos I, Espinosa M, Rivera F, Oliet A, Fernández-Vega F, Praga M, Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Española de Nefrología : Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol 21: 697–704, 2010 - PMC - PubMed
-
- Cattran DC, Pei Y, Greenwood CM, Ponticelli C, Passerini P, Honkanen E: Validation of a predictive model of idiopathic membranous nephropathy: Its clinical and research implications. Kidney Int 51: 901–907, 1997 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

