Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle
- PMID: 24610934
- PMCID: PMC4116419
- DOI: 10.1152/ajplung.00155.2013
Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle
Abstract
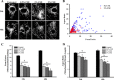
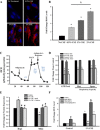
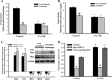
The balance between mitochondrial fission and fusion is crucial for mitochondria to perform its normal cellular functions. We hypothesized that cigarette smoke (CS) disrupts this balance and enhances mitochondrial dysfunction in the airway. In nonasthmatic human airway smooth muscle (ASM) cells, CS extract (CSE) induced mitochondrial fragmentation and damages their networked morphology in a concentration-dependent fashion, via increased expression of mitochondrial fission protein dynamin-related protein 1 (Drp1) and decreased fusion protein mitofusin (Mfn) 2. CSE effects on Drp1 vs. Mfn2 and mitochondrial network morphology involved reactive oxygen species (ROS), activation of extracellular signal-regulated kinase (ERK), phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt), protein kinase C (PKC) and proteasome pathways, as well as transcriptional regulation via factors such as NF-κB and nuclear erythroid 2-related factor 2. Inhibiting Drp1 prevented CSE effects on mitochondrial networks and ROS generation, whereas blocking Mfn2 had the opposite, detrimental effect. In ASM from asmatic patients, mitochondria exhibited substantial morphological defects at baseline and showed increased Drp1 but decreased Mfn2 expression, with exacerbating effects of CSE. Overall, these results highlight the importance of mitochondrial networks and their regulation in the context of cellular changes induced by insults such as inflammation (as in asthma) or CS. Altered mitochondrial fission/fusion proteins have a further potential to influence parameters such as ROS and cell proliferation and apoptosis relevant to airway diseases.
Keywords: asthma; dynamin-related protein 1; lung; mitochondria; mitofusin 2; signaling.
Figures






Comment in
-
Exposure of airway smooth muscle cells to cigarette smoke extract.Am J Physiol Lung Cell Mol Physiol. 2014 Aug 15;307(4):L345. doi: 10.1152/ajplung.00161.2014. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25128538 No abstract available.
-
Response to letter by Dr. Marc Hershenson (exposure of airway smooth muscle cells to cigarette smoke extract).Am J Physiol Lung Cell Mol Physiol. 2014 Aug 15;307(4):L346. doi: 10.1152/ajplung.00194.2014. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25128539 Free PMC article. No abstract available.
References
-
- Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol 294: H570–H578, 2008 - PubMed
-
- Baglole CJ, Bushinsky SM, Garcia TM, Kode A, Rahman I, Sime PJ, Phipps RP. Differential induction of apoptosis by cigarette smoke extract in primary human lung fibroblast strains: implications for emphysema. Am J Physiol Lung Cell Mol Physiol 291: L19–L29, 2006 - PubMed
-
- Bargagli E, Olivieri C, Bennett D, Prasse A, Muller-Quernheim J, Rottoli P. Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir Med 103: 1245–1256, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

