Up-regulation of β-amyloidogenesis in neuron-like human cells by both 24- and 27-hydroxycholesterol: protective effect of N-acetyl-cysteine
- PMID: 24612036
- PMCID: PMC4326893
- DOI: 10.1111/acel.12206
Up-regulation of β-amyloidogenesis in neuron-like human cells by both 24- and 27-hydroxycholesterol: protective effect of N-acetyl-cysteine
Abstract
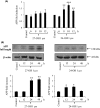
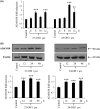
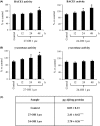
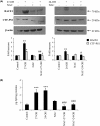
An abnormal accumulation of cholesterol oxidation products in the brain of patients with Alzheimer's disease (AD) would further link an impaired cholesterol metabolism in the pathogenesis of the disease. The first evidence stemming from the content of oxysterols in autopsy samples from AD and normal brains points to an increase in both 27-hydroxycholesterol (27-OH) and 24-hydroxycholesterol (24-OH) in the frontal cortex of AD brains, with a trend that appears related to the disease severity. The challenge of differentiated SK-N-BE human neuroblastoma cells with patho-physiologically relevant amounts of 27-OH and 24-OH showed that both oxysterols induce a net synthesis of Aβ1-42 by up-regulating expression levels of amyloid precursor protein and β-secretase, as well as the β-secretase activity. Interestingly, cell pretreatment with N-acetyl-cysteine (NAC) fully prevented the enhancement of β-amyloidogenesis induced by the two oxysterols. The reported findings link an impaired cholesterol oxidative metabolism to an excessive β-amyloidogenesis and point to NAC as an efficient inhibitor of oxysterols-induced Aβ toxic peptide accumulation in the brain.
Keywords: 24-hydroxycholesterol; 27-hydroxycholesterol; Alzheimer's disease; BACE1; N-acetyl-cysteine; amyloid β.
© 2014 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures


Similar articles
-
Interaction between 24-hydroxycholesterol, oxidative stress, and amyloid-β in amplifying neuronal damage in Alzheimer's disease: three partners in crime.Aging Cell. 2011 Jun;10(3):403-17. doi: 10.1111/j.1474-9726.2011.00681.x. Epub 2011 Apr 5. Aging Cell. 2011. PMID: 21272192
-
22R-Hydroxycholesterol protects neuronal cells from beta-amyloid-induced cytotoxicity by binding to beta-amyloid peptide.J Neurochem. 2002 Dec;83(5):1110-9. doi: 10.1046/j.1471-4159.2002.01202.x. J Neurochem. 2002. PMID: 12437582
-
A silver lining for 24-hydroxycholesterol in Alzheimer's disease: The involvement of the neuroprotective enzyme sirtuin 1.Redox Biol. 2018 Jul;17:423-431. doi: 10.1016/j.redox.2018.05.009. Epub 2018 May 22. Redox Biol. 2018. PMID: 29883958 Free PMC article.
-
Oxysterols and Alzheimer's disease.Acta Neurol Scand Suppl. 2006;185:43-9. doi: 10.1111/j.1600-0404.2006.00684.x. Acta Neurol Scand Suppl. 2006. PMID: 16866910 Review.
-
Alterations in cholesterol metabolism as a risk factor for developing Alzheimer's disease: Potential novel targets for treatment.J Steroid Biochem Mol Biol. 2019 Jun;190:104-114. doi: 10.1016/j.jsbmb.2019.03.003. Epub 2019 Mar 13. J Steroid Biochem Mol Biol. 2019. PMID: 30878503 Review.
Cited by
-
27-Hydroxycholesterol in cancer development and drug resistance.J Enzyme Inhib Med Chem. 2025 Dec;40(1):2507670. doi: 10.1080/14756366.2025.2507670. Epub 2025 May 22. J Enzyme Inhib Med Chem. 2025. PMID: 40401382 Free PMC article. Review.
-
Positive Feedback Loops in Alzheimer's Disease: The Alzheimer's Feedback Hypothesis.J Alzheimers Dis. 2018;66(1):25-36. doi: 10.3233/JAD-180583. J Alzheimers Dis. 2018. PMID: 30282364 Free PMC article. Review.
-
Oxysterols and Oxysterol Sulfates in Alzheimer's Disease Brain and Cerebrospinal Fluid.J Alzheimers Dis. 2022;87(4):1527-1536. doi: 10.3233/JAD-220083. J Alzheimers Dis. 2022. PMID: 35491790 Free PMC article.
-
Oxysterol levels and metabolism in the course of neuroinflammation: insights from in vitro and in vivo models.J Neuroinflammation. 2018 Mar 9;15(1):74. doi: 10.1186/s12974-018-1114-8. J Neuroinflammation. 2018. PMID: 29523207 Free PMC article.
-
Oxidative Stress and Beta Amyloid in Alzheimer's Disease. Which Comes First: The Chicken or the Egg?Antioxidants (Basel). 2021 Sep 16;10(9):1479. doi: 10.3390/antiox10091479. Antioxidants (Basel). 2021. PMID: 34573112 Free PMC article. Review.
References
-
- Alexandrov P, Cui JG, Zhao Y, Lukiw WJ. 24S-hydroxycholesterol induces inflammatory gene expression in primary human neural cells. NeuroReport. 2005;16:909–913. - PubMed
-
- Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol. Sci. 2013;34:167–177. - PubMed
-
- Biasi F, Mascia C, Astegiano M, Chiarpotto E, Nano M, Vizio B, Leonarduzzi G, Poli G. Pro-oxidant and proapoptotic effects of cholesterol oxidation products on human colonic epithelial cells: a potential mechanism of inflammatory bowel disease progression. Free Radic. Biol. Med. 2009;47:1731–1741. - PubMed
-
- Biasi F, Guina T, Maina M, Cabboi B, Deiana M, Tuberoso CI, Calfapietra S, Chiarpotto E, Sottero B, Gamba P, Gargiulo S, Brunetto V, Testa G, Dessì MA, Poli G, Leonarduzzi G. Phenolic compounds present in Sardinian wine extracts protect against the production of inflammatory cytokines induced by oxysterols in CaCo-2 human enterocyte-like cells. Biochem. Pharmacol. 2013;86:138–145. - PubMed
-
- Björkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004;24:806–815. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

