Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart
- PMID: 24612461
- PMCID: PMC4040127
- DOI: 10.1111/acel.12203
Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart
Abstract
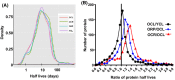
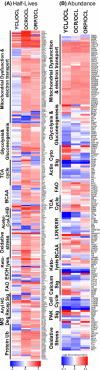
Chronic caloric restriction (CR) and rapamycin inhibit the mechanistic target of rapamycin (mTOR) signaling, thereby regulating metabolism and suppressing protein synthesis. Caloric restriction or rapamycin extends murine lifespan and ameliorates many aging-associated disorders; however, the beneficial effects of shorter treatment on cardiac aging are not as well understood. Using a recently developed deuterated-leucine labeling method, we investigated the effect of short-term (10 weeks) CR or rapamycin on the proteomics turnover and remodeling of the aging mouse heart. Functionally, we observed that short-term CR and rapamycin both reversed the pre-existing age-dependent cardiac hypertrophy and diastolic dysfunction. There was no significant change in the cardiac global proteome (823 proteins) turnover with age, with a median half-life 9.1 days in the 5-month-old hearts and 8.8 days in the 27-month-old hearts. However, proteome half-lives of old hearts significantly increased after short-term CR (30%) or rapamycin (12%). This was accompanied by attenuation of age-dependent protein oxidative damage and ubiquitination. Quantitative proteomics and pathway analysis revealed an age-dependent decreased abundance of proteins involved in mitochondrial function, electron transport chain, citric acid cycle, and fatty acid metabolism as well as increased abundance of proteins involved in glycolysis and oxidative stress response. This age-dependent cardiac proteome remodeling was significantly reversed by short-term CR or rapamycin, demonstrating a concordance with the beneficial effect on cardiac physiology. The metabolic shift induced by rapamycin was confirmed by metabolomic analysis.
Keywords: caloric restriction; cardiac aging; dynamics; proteomics; rapamycin.
© 2014 The Authors. Aging Cell Published by the Anatomical Society and John Wiley & Sons Ltd.
Figures




References
-
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Invest. 2009;119:573–581. - PMC - PubMed
-
- Breusing N, Grune T. Regulation of proteasome-mediated protein degradation during oxidative stress and aging. Biol. Chem. 2008;389:203–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

