Angiotensin receptor type 1 and endothelin receptor type A on immune cells mediate migration and the expression of IL-8 and CCL18 when stimulated by autoantibodies from systemic sclerosis patients
- PMID: 24612997
- PMCID: PMC4060229
- DOI: 10.1186/ar4503
Angiotensin receptor type 1 and endothelin receptor type A on immune cells mediate migration and the expression of IL-8 and CCL18 when stimulated by autoantibodies from systemic sclerosis patients
Abstract
Introduction: Agonistic autoantibodies (Aabs) against the angiotensin II receptor type 1 (AT1R) and the endothelin receptor type A (ETAR) have been identified in patients with systemic sclerosis (SSc). In our present study, we examined the expression of the AT1R and the ETAR in human immune cells and the pathological effects mediated through these receptors by their corresponding Aabs.
Methods: Protein expression of AT1R and ETAR on peripheral blood mononuclear cells (PBMCs) from healthy individuals and SSc patients was analyzed using flow cytometry, and mRNA expression of both receptors in PBMCs from healthy donors was examined by real-time PCR. In addition, PBMCs from healthy donors were stimulated in vitro with affinity-purified immunoglobulin G (IgG) fractions from SSc patients positive for AT1R and ETAR Aabs, as well as with IgG from healthy donors serving as controls. Alterations in cell surface marker expression, cytokine secretion and chemotactic motility were analyzed using flow cytometry, enzyme-linked immunosorbent assays and chemotaxis assays, respectively. The results were correlated with the characteristics and clinical findings of the IgG donors.
Results: Both AT1R and ETAR were expressed on PBMCs in humans. Protein expression of both receptors was decreased in SSc patients compared with that of healthy donors and declined during the course of disease. IgG fractions of SSc patients positive for AT1R and ETAR Aabs induced T-cell migration in an Aab level-dependent manner. Moreover, IgG of SSc patients stimulated PBMCs to produce more interleukin 8 (IL-8) and chemokine (C-C motif) ligand 18 (CCL18) than did the IgG of healthy donors. All effects were significantly reduced by selective AT1R and ETAR antagonists. Statistical analysis revealed an association of SSc-IgG induced high IL-8 concentrations with an early disease stage and of high CCL18 concentrations with lung fibrosis onset and vascular complications in the respective IgG donors.
Conclusion: In our present study, we could demonstrate the expression of both AT1R and ETAR on human peripheral T cells, B cells and monocytes. The decreased receptor expression in SSc patients, the inflammatory and profibrotic effects upon Aab stimulation of PBMCs in vitro and the associations with clinical findings suggest a role for Aab-induced activation of immune cells mediated by the AT1R and the ETAR in the pathogenesis or even the onset of the disease.
Figures



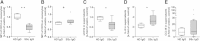



References
-
- Sakkas LI, Chikanza IC, Platsoucas CD. Mechanisms of disease: the role of immune cells in the pathogenesis of systemic sclerosis. Nat Clin Pract Rheumatol. 2006;2:679–685. - PubMed
-
- Riemekasten G, Philippe A, Näther M, Slowinski T, Müller DN, Heidecke H, Matucci-Cerinic M, Czirják L, Lukitsch I, Becker M, Kill A, van Laar JM, Catar R, Luft FC, Burmester GR, Hegner B, Dragun D. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann Rheum Dis. 2011;70:530–536. doi: 10.1136/ard.2010.135772. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

