Transmission of mitochondrial mutations and action of purifying selection in Drosophila melanogaster
- PMID: 24614071
- PMCID: PMC4091738
- DOI: 10.1038/ng.2919
Transmission of mitochondrial mutations and action of purifying selection in Drosophila melanogaster
Abstract
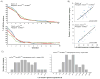
It is not known how selection affects mutations in the multiple copies of the mitochondrial genome. We transferred cytoplasm between D. melanogaster embryos carrying mitochondrial mutations to create heteroplasmic lines transmitting two mitochondrial genotypes. Increased temperature imposed selection against a temperature-sensitive mutation affecting cytochrome oxidase, driving decreases in the abundance of the mutant genome over successive generations. Selection did not influence the health or fertility of the flies but acted during midoogenesis to influence competition between the genomes. Mitochondria might incur an advantage through selective localization, survival or proliferation, yet timing and insensitivity to park mutation suggest that preferential proliferation underlies selection. Selection drove complete replacement of the temperature-sensitive mitochondrial genome by a wild-type genome but also stabilized the multigenerational transmission of two genomes carrying complementing detrimental mutations. While they are so balanced, these stably transmitted mutations have no detrimental phenotype, but their segregation could contribute to disease phenotypes and somatic aging.
Figures

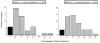

References
-
- Chinnery PF, et al. The inheritance of mitochondrial DNA heteroplasmy: random drift, selection or both? Trends Genet. 2000;16:500–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

