An autoreactive antibody from an SLE/HIV-1 individual broadly neutralizes HIV-1
- PMID: 24614107
- PMCID: PMC3973118
- DOI: 10.1172/JCI73441
An autoreactive antibody from an SLE/HIV-1 individual broadly neutralizes HIV-1
Abstract
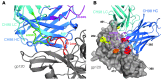
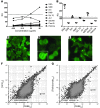
Broadly HIV-1-neutralizing antibodies (BnAbs) display one or more unusual traits, including a long heavy chain complementarity-determining region 3 (HCDR3), polyreactivity, and high levels of somatic mutations. These shared characteristics suggest that BnAb development might be limited by immune tolerance controls. It has been postulated that HIV-1-infected individuals with autoimmune disease and defective immune tolerance mechanisms may produce BnAbs more readily than those without autoimmune diseases. In this study, we identified an HIV-1-infected individual with SLE who exhibited controlled viral load (<5,000 copies/ml) in the absence of controlling HLA phenotypes and developed plasma HIV-1 neutralization breadth. We collected memory B cells from this individual and isolated a BnAb, CH98, that targets the CD4 binding site (CD4bs) of HIV-1 envelope glycoprotein 120 (gp120). CH98 bound to human antigens including dsDNA, which is specifically associated with SLE. Anti-dsDNA reactivity was also present in the patient's plasma. CH98 had a mutation frequency of 25% and 15% nt somatic mutations in the heavy and light chain variable domains, respectively, a long HCDR3, and a deletion in the light chain CDR1. The occurrence of anti-dsDNA reactivity by a HIV-1 CD4bs BnAb in an individual with SLE raises the possibility that some BnAbs and SLE-associated autoantibodies arise from similar pools of B cells.
Figures


References
-
- McInerney TL, McLain L, Armstrong SJ, Dimmock NJ. A human IgG1 (b12) specific for the CD4 binding site of HIV-1 neutralizes by inhibiting the virus fusion entry process, but b12 Fab neutralizes by inhibiting a postfusion event. Virology. 1997;233(2):313–326. doi: 10.1006/viro.1997.8547. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

