Extracellular vesicles in luminal fluid of the ovine uterus
- PMID: 24614226
- PMCID: PMC3948691
- DOI: 10.1371/journal.pone.0090913
Extracellular vesicles in luminal fluid of the ovine uterus
Abstract
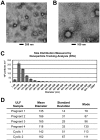
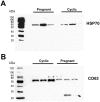
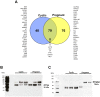
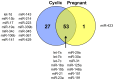
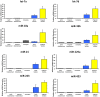
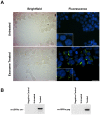
Microvesicles and exosomes are nanoparticles released from cells and can contain small RNAs, mRNA and proteins that affect cells at distant sites. In sheep, endogenous beta retroviruses (enJSRVs) are expressed in the endometrial epithelia of the uterus and can be transferred to the conceptus trophectoderm. One potential mechanism of enJSRVs transfer from the uterus to the conceptus is via exosomes/microvesicles. Therefore, studies were conducted to evaluate exosomes in the uterine luminal fluid (ULF) of sheep. Exosomes/microvesicles (hereafter referred to as extracellular vesicles) were isolated from the ULF of day 14 cyclic and pregnant ewes using ExoQuick-TC. Transmission electron microscopy and nanoparticle tracking analysis found the isolates contained vesicles that ranged from 50 to 200 nm in diameter. The isolated extracellular vesicles were positive for two common markers of exosomes (CD63 and HSP70) by Western blot analysis. Proteins in the extracellular vesicles were determined by mass spectrometry and Western blot analysis. Extracellular vesicle RNA was analyzed for small RNAs by sequencing and enJSRVs RNA by RT-PCR. The ULF extracellular vesicles contained a large number of small RNAs and miRNAs including 81 conserved mature miRNAs. Cyclic and pregnant ULF extracellular vesicles contained enJSRVs env and gag RNAs that could be delivered to heterologous cells in vitro. These studies support the hypothesis that ULF extracellular vesicles can deliver enJSRVs RNA to the conceptus, which is important as enJSRVs regulate conceptus trophectoderm development. Importantly, these studies support the idea that extracellular vesicles containing select miRNAs, RNAs and proteins are present in the ULF and likely have a biological role in conceptus-endometrial interactions important for the establishment and maintenance of pregnancy.
Conflict of interest statement
Figures

References
-
- Spencer TE, Sandra O, Wolf E (2008) Genes involved in conceptus-endometrial interactions in ruminants: insights from reductionism and thoughts on holistic approaches. Reproduction 135: 165–179. - PubMed
-
- Spencer TE, Johnson GA, Bazer FW, Burghardt RC, Palmarini M (2007) Pregnancy recognition and conceptus implantation in domestic ruminants: roles of progesterone, interferons and endogenous retroviruses. Reprod Fertil Dev 19: 65. - PubMed
-
- Guillomot M (1995) Cellular interactions during implantation in domestic ruminants. J Reprod Fertil Suppl 49: 39–51. - PubMed
-
- Fléchon JE, Guillomot M, Charlier M, Fléchon B, Martal J (1986) Experimental studies on the elongation of the ewe blastocyst. Reprod Nutr Dev 26: 1017–1024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

