Inhibition of dopamine signaling suppresses cGMP accumulation in rd1 retinal organ cultures
- PMID: 24614363
- PMCID: PMC4275426
- DOI: 10.1097/WNR.0000000000000145
Inhibition of dopamine signaling suppresses cGMP accumulation in rd1 retinal organ cultures
Abstract
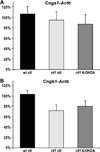
The rd1 mouse is a model of retinitis pigmentosa, an inherited photoreceptor neurodegenerative disease. In rd1 retina, early onset rod degeneration is caused by a Pde6b mutation that leads to high levels of intracellular cyclic guanosine monophosphate (cGMP). Cyclic nucleotide-gated ion channels (CNGCs), necessary for phototransduction, are regulated by cGMP. We have previously demonstrated that inhibition of dopamine signaling blocks rd1 photoreceptor degeneration in retinal organ cultures. The mechanism underlying this protection remains unknown. The aim of this study was to determine whether inhibition of dopamine signaling alters cGMP accumulation or CNGC expression. Dopamine depletion from rd1 retinal organ cultures resulted in a significant decrease in cGMP compared with untreated rd1 organ cultures. However, cGMP levels in both treated and untreated rd1 organ cultures significantly exceeded cGMP levels in wild-type (wt) retinal organ cultures. The CNGC expression profile was first determined in vivo. Both channel subunits, Cnga1 and Cngb1, are expressed at low levels by postnatal day 2 (P2), increasing sharply by P6 with a modest increase after P12 in wt retina. A similar pattern is seen in rd1 retina until P12 when expression levels decrease, leading to cell death. No significant difference was observed in the expression of either Cnga1 or Cngb1 in organ cultures from wt, rd1, and dopamine-depleted rd1 retinas. Our results show that dopamine depletion significantly decreases cGMP levels in rd1 retinal organ cultures, but that cGMP accumulation remains high, requiring additional mechanisms for photoreceptor protection. These mechanisms may include activation of protein kinase G-signaling pathways and/or crosstalk with dopamine signaling through cyclic adenosine monophosphate pathways.
Conflict of interest statement
Conflicts of interest: None.
Figures


References
-
- Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–680. - PubMed
-
- Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiological reviews. 2002;82:769–824. - PubMed
-
- Farber DB, Flannery JG, Bowes-Rickman C. The rd Mouse Story: Seventy Years of Research on an Animal Model of Inherited Retinal Degeneration. Prog Retin Eye Res. 1994;13:31–64.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

