Characterization of the population pharmacokinetics of ampicillin in neonates using an opportunistic study design
- PMID: 24614374
- PMCID: PMC4068432
- DOI: 10.1128/AAC.02374-13
Characterization of the population pharmacokinetics of ampicillin in neonates using an opportunistic study design
Abstract
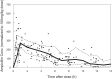
Although ampicillin is the most commonly used drug in neonates, developmental pharmacokinetic (PK) data to guide dosing are lacking. Ampicillin is primarily renally eliminated, and developmental changes are expected to influence PK. We conducted an open-label, multicenter, opportunistic, prospective PK study of ampicillin in neonates stratified by gestational age (GA) (≤ 34 or >34 weeks) and postnatal age (PNA) (≤ 7 or >7 days). Drug concentrations were measured by tandem mass spectrometry. PK data were analyzed using population nonlinear mixed-effects modeling in NONMEM 7.2. Monte Carlo simulations were conducted to determine the probability of target attainment for the time in which the total steady-state ampicillin concentrations remained above the MIC (T>MIC) for 50%, 75%, and 100% of the dosing interval. A total of 142 PK samples from 73 neonates were analyzed (median [range] GA, 36 [24 to 41] weeks; PNA, 5 [0 to 25] days). The median ampicillin dose was 200 (100 to 350) mg/kg/day. Postmenstrual age and serum creatinine were covariates for ampicillin clearance (CL). A simplified dosing regimen of 50 mg/kg every 12 h for GA of ≤ 34 weeks and PNA of ≤ 7 days, 75 mg/kg every 12 h for GA of ≤ 34 weeks and PNA of ≥ 8 and ≤ 28 days, and 50 mg/kg every 8 h for GA of >34 weeks and PNA of ≤ 28 days achieved the prespecified surrogate efficacy target in 90% of simulated subjects. Ampicillin CL was associated with neonatal development. A simplified dosing regimen stratified by GA and PNA achieves the desired surrogate therapeutic target in the vast majority of neonates.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Thomson Reuters Clinical Editorial Staff. 2011. Neofax®, 24th ed. Thomson Reuters, Hoboken, NJ
-
- Axline SG, Yaffe SJ, Simon HJ. 1967. Clinical pharmacology of antimicrobials in premature infants. II. Ampicillin, methicillin, oxacillin, neomycin, and colistin. Pediatrics 39:97–107 - PubMed
-
- Barrons RW, Murray KM, Richey RM. 1992. Populations at risk for penicillin-induced seizures. Ann. Pharmacother. 26:26–29 - PubMed
-
- Boe RW, Williams CP, Bennett JV, Oliver TK., Jr 1967. Serum levels of methicillin and ampicillin in newborn and premature infants in relation to postnatal age. Pediatrics 39:194–201 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN267200700051C/HD/NICHD NIH HHS/United States
- K24 HD058735/HD/NICHD NIH HHS/United States
- 1U01FD004858-01/FD/FDA HHS/United States
- U10 HD045934 05/HD/NICHD NIH HHS/United States
- 1K23HD064814/HD/NICHD NIH HHS/United States
- K23 HD068497/HD/NICHD NIH HHS/United States
- HHSN275201000003C/HD/NICHD NIH HHS/United States
- K23 HD044799/HD/NICHD NIH HHS/United States
- UL1TR001117/TR/NCATS NIH HHS/United States
- K23HD068497/HD/NICHD NIH HHS/United States
- HHSN275201000003I/HD/NICHD NIH HHS/United States
- 1K24HD058735-05/HD/NICHD NIH HHS/United States
- UL1 TR001117/TR/NCATS NIH HHS/United States
- U54 HD071600/HD/NICHD NIH HHS/United States
- HHSN275201000001Z/HD/NICHD NIH HHS/United States
- U54 HD071600-01/HD/NICHD NIH HHS/United States
- R01 HD057956/HD/NICHD NIH HHS/United States
- U01 FD004858/FD/FDA HHS/United States
- K23 HD064814/HD/NICHD NIH HHS/United States
- U10 HD045934/HD/NICHD NIH HHS/United States
- 1R01HD057956-05/HD/NICHD NIH HHS/United States
- HHSN275201000001G/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

