Determination of vancomycin pharmacokinetics in neonates to develop practical initial dosing recommendations
- PMID: 24614381
- PMCID: PMC3993213
- DOI: 10.1128/AAC.01718-13
Determination of vancomycin pharmacokinetics in neonates to develop practical initial dosing recommendations
Abstract
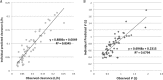
Variability in neonatal vancomycin pharmacokinetics and the lack of consensus for optimal trough concentrations in neonatal intensive care units pose challenges to dosing vancomycin in neonates. Our objective was to determine vancomycin pharmacokinetics in neonates and evaluate dosing regimens to identify whether practical initial recommendations that targeted trough concentrations most commonly used in neonatal intensive care units could be determined. Fifty neonates who received vancomycin with at least one set of steady-state levels were evaluated retrospectively. Mean pharmacokinetic values were determined using first-order pharmacokinetic equations, and Monte Carlo simulation was used to evaluate initial dosing recommendations for target trough concentrations of 15 to 20 mg/liter, 5 to 20 mg/liter, and ≤20 mg/liter. Monte Carlo simulation revealed that dosing by mg/kg of body weight was optimal where intermittent dosing of 9 to 12 mg/kg intravenously (i.v.) every 8 h (q8h) had the highest probability of attaining a target trough concentration of 15 to 20 mg/liter. However, continuous infusion with a loading dose of 10 mg/kg followed by 25 to 30 mg/kg per day infused over 24 h had the best overall probability of target attainment. Initial intermittent dosing of 9 to 15 mg/kg i.v. q12h was optimal for target trough concentrations of 5 to 20 mg/liter and ≤20 mg/liter. In conclusion, we determined that the practical initial vancomycin dose of 10 mg/kg vancomycin i.v. q12h was optimal for vancomycin trough concentrations of either 5 to 20 mg/liter or ≤20 mg/liter and that the same initial dose q8h was optimal for target trough concentrations of 15 to 20 mg/liter. However, due to large interpatient vancomycin pharmacokinetic variability in neonates, monitoring of serum concentrations is recommended when trough concentrations between 15 and 20 mg/liter or 5 and 20 mg/liter are desired.
Figures
References
-
- Stoll BJ, Hansen N, Fanaraoff AA, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK. 2002. Late-onset sepsis in very low birth weight neonates: the experiences of the NICHD Neonatal Research Network. Pediatrics 110:285–291. 10.1542/peds.110.2.285 - DOI - PubMed
-
- Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwiwtz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52:285–292. 10.1093/cid/cir034 - DOI - PubMed
-
- Rybak M, Lomaestro B, Rotschafer J, Moellering R, Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 66:82–98. 10.2146/ajhp080434 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical