Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study
- PMID: 24614494
- PMCID: PMC4195975
- DOI: 10.1038/mp.2014.9
Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study
Abstract
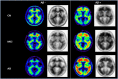
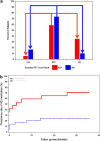
This study was designed to evaluate whether subjects with amyloid beta (Aβ) pathology, detected using florbetapir positron emission tomorgraphy (PET), demonstrated greater cognitive decline than subjects without Aβ pathology. Sixty-nine cognitively normal (CN) controls, 52 with recently diagnosed mild cognitive impairment (MCI) and 31 with probable Alzheimer's disease (AD) dementia were included in the study. PET images obtained in these subjects were visually rated as positive (Aβ+) or negative (Aβ-), blind to diagnosis. Fourteen percent (10/69) of CN, 37% (19/52) of MCI and 68% (21/31) of AD were Aβ+. The primary outcome was change in ADAS-Cog score in MCI subjects after 36 months; however, additional outcomes included change on measures of cognition, function and diagnostic status. Aβ+ MCI subjects demonstrated greater worsening compared with Aβ- subjects on the ADAS-Cog over 36 months (5.66 ± 1.47 vs -0.71 ± 1.09, P = 0.0014) as well as on the mini-mental state exam (MMSE), digit symbol substitution (DSS) test, and a verbal fluency test (P < 0.05). Similar to MCI subjects, Aβ+ CN subjects showed greater decline on the ADAS-Cog, digit-symbol-substitution test and verbal fluency (P<0.05), whereas Aβ+ AD patients showed greater declines in verbal fluency and the MMSE (P < 0.05). Aβ+ subjects in all diagnostic groups also showed greater decline on the CDR-SB (P<0.04), a global clinical assessment. Aβ+ subjects did not show significantly greater declines on the ADCS-ADL or Wechsler Memory Scale. Overall, these findings suggest that in CN, MCI and AD subjects, florbetapir PET Aβ+ subjects show greater cognitive and global deterioration over a 3-year follow-up than Aβ- subjects do.
Figures

References
-
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. - PMC - PubMed
-
- Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18 ((4 Suppl:S1–S2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

