Intraperitoneal injection of the pancreatic peptide amylin potently reduces behavioral impairment and brain amyloid pathology in murine models of Alzheimer's disease
- PMID: 24614496
- PMCID: PMC4161670
- DOI: 10.1038/mp.2014.17
Intraperitoneal injection of the pancreatic peptide amylin potently reduces behavioral impairment and brain amyloid pathology in murine models of Alzheimer's disease
Abstract
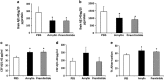
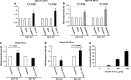
Amylin, a pancreatic peptide, and amyloid-beta peptides (Aβ), a major component of Alzheimer's disease (AD) brain, share similar β-sheet secondary structures, but it is not known whether pancreatic amylin affects amyloid pathogenesis in the AD brain. Using AD mouse models, we investigated the effects of amylin and its clinical analog, pramlintide, on AD pathogenesis. Surprisingly, chronic intraperitoneal (i.p.) injection of AD animals with either amylin or pramlintide reduces the amyloid burden as well as lowers the concentrations of Aβ in the brain. These treatments significantly improve their learning and memory assessed by two behavioral tests, Y maze and Morris water maze. Both amylin and pramlintide treatments increase the concentrations of Aβ1-42 in cerebral spinal fluid (CSF). A single i.p. injection of either peptide also induces a surge of Aβ in the serum, the magnitude of which is proportionate to the amount of Aβ in brain tissue. One intracerebroventricular injection of amylin induces a more significant surge in serum Aβ than one i.p. injection of the peptide. In 330 human plasma samples, a positive association between amylin and Aβ1-42 as well as Aβ1-40 is found only in patients with AD or amnestic mild cognitive impairment. As amylin readily crosses the blood-brain barrier, our study demonstrates that peripheral amylin's action on the central nervous system results in translocation of Aβ from the brain into the CSF and blood that could be an explanation for a positive relationship between amylin and Aβ in blood. As naturally occurring amylin may play a role in regulating Aβ in brain, amylin class peptides may provide a new avenue for both treatment and diagnosis of AD.
Figures



References
-
- Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 1998;19:883–889. - PubMed
-
- Olsson M, Herrington MK, Reidelberger RD, Permert J, Arnelo U. Comparison of the effects of chronic central administration and chronic peripheral administration of islet amyloid polypeptide on food intake and meal pattern in the rat. Peptides. 2007;28:1416–1423. - PubMed
-
- Roth JD, Roth JD, Erickson MR, Chen S, Parkes DG. Amylin and the regulation of appetite and adiposity: recent advances in receptor signaling, neurobiology and pharmacology GLP-1R and amylin agonism in metabolic disease: complementary mechanisms and future opportunities. Curr Opin Endocrinol Diabetes Obes. 2013;20:8–13. - PubMed
-
- Westfall TC, Curfman-Falvey M. Amylin-induced relaxation of the perfused mesenteric arterial bed: meditation by calcitonin gene-related peptide receptors. J Cardiovasc Pharmacol. 1995;26:932–936. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

