Assessment of cancer therapy-induced oral mucositis using a patient-reported oral mucositis experience questionnaire
- PMID: 24614512
- PMCID: PMC3948915
- DOI: 10.1371/journal.pone.0091733
Assessment of cancer therapy-induced oral mucositis using a patient-reported oral mucositis experience questionnaire
Abstract
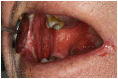
Objectives: Treatment of oral mucositis (OM) is challenging. In order to develop and test useful treatment approaches, the development of reliable, reproducible and simpler methods than are currently available for assessment of OM is important. A Patient-Reported Oral Mucositis Symptom (PROMS) scale was assessed in patients with head and neck cancer to determine if the patient-reported OM experience, as determined by using the PROMS scale, correlate with OM assessed by clinician-based scoring tools.
Materials and methods: Fifty patients with head and neck cancer and undergoing radiotherapy consented to participate. They were examined before cancer treatment and twice weekly during 6-7 weeks of therapy and once 4-6 weeks after therapy. Signs of OM were evaluated using the 3 clinician-based scoring tools; NCI-CTCAE v.3, the OMAS criteria and the Total VAS-OMAS. The participants' OM experiences were recorded using PROMS-questionnaires consisting of 10 questions on a visual analogue scale. Spearman rank correlation test were applied between the PROMS scale values and the clinician-determined scores. Repeated measures mixed linear models were applied to appraise the strengths of correlation at the different time points throughout the observation period.
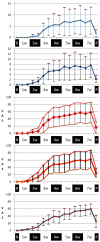
Results: Thirty-three participants completed all stages of the study. The participant experience of OM using the PROMS scale demonstrates good correlations (Spearman's Rho 0.65-0.78, p<0.001) with the clinician-determined scores on the group level over all time points and poor to good correlations (Spearman's Rho -0.12-0.70, p<0.001) on the group level at different time points during and after therapy. When mouth opening was problematic, i.e. during the 6th and 7th week after commencing cancer treatment, the Spearman's Rho varied between 0.19 and 0.70 (p<0.001).
Conclusion: Patient experience of OM, as reported by the PROMS scale may be a feasible substitute for clinical assessment in situations where patients cannot endure oral examinations.
Conflict of interest statement
Figures


References
-
- Epstein JB, Thariat J, Bensadoun RJ, Barasch A, Murphy BA, et al. (2012) Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin 62: 400–422. - PubMed
-
- Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, et al. (2003) Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiation therapy with or without chemotherapy: a systematic literature review. Radiother Oncol 66: 253–262. - PubMed
-
- Davies AN, Epstein JB (2010) Oral complications of Cancer and its Management. Oxford, UK: Oxford University Press. 324 p.
-
- Elting LS, Cooksley CD, Chambers MS, Garden AS (2007) Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys 68: 1110–1120. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

