The plasma protein fibrinogen stabilizes clusters of red blood cells in microcapillary flows
- PMID: 24614613
- PMCID: PMC3949293
- DOI: 10.1038/srep04348
The plasma protein fibrinogen stabilizes clusters of red blood cells in microcapillary flows
Abstract
The supply of oxygen and nutrients and the disposal of metabolic waste in the organs depend strongly on how blood, especially red blood cells, flow through the microvascular network. Macromolecular plasma proteins such as fibrinogen cause red blood cells to form large aggregates, called rouleaux, which are usually assumed to be disaggregated in the circulation due to the shear forces present in bulk flow. This leads to the assumption that rouleaux formation is only relevant in the venule network and in arterioles at low shear rates or stasis. Thanks to an excellent agreement between combined experimental and numerical approaches, we show that despite the large shear rates present in microcapillaries, the presence of either fibrinogen or the synthetic polymer dextran leads to an enhanced formation of robust clusters of red blood cells, even at haematocrits as low as 1%. Robust aggregates are shown to exist in microcapillaries even for fibrinogen concentrations within the healthy physiological range. These persistent aggregates should strongly affect cell distribution and blood perfusion in the microvasculature, with putative implications for blood disorders even within apparently asymptomatic subjects.
Figures


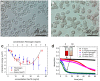
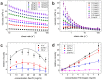
References
-
- Fahraeus R. The Influence of the rouleau formation of the erythrocytes on the rheology of the blood. Acta Med. Scand. 161, 151–165 (1958). - PubMed
-
- Merrill E. W., Cokelet G. C., Britten A. & Wells R. E. Non-Newtonian rheology of human blood - effect of fibrinogen deduced by “subtraction”. Circ Res. 13, 48–55 (1963). - PubMed
-
- Bishop J. J., Popel A. S., Intaglietta M. & Johnson P. C. Rheological effects of red blood cell aggregation in the venous network: A review of recent studies. Biorheology 38, 263–274 (2001). - PubMed
-
- Marton Zs., Kesmarky G., Vekasi J., Cser A., Russai R., Horvath B. & Toth K. Red blood cell aggregation measurements in whole blood and in fibrinogen solutions by different methods. Clin. Hemorheol. Micro. 24, 75–83 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

