Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease
- PMID: 24615270
- PMCID: PMC8966042
- DOI: 10.1002/14651858.CD010115.pub2
Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease
Abstract
Background: Inhaled corticosteroids (ICS) are anti-inflammatory drugs that have proven benefits for people with worsening symptoms of chronic obstructive pulmonary disease (COPD) and repeated exacerbations. They are commonly used as combination inhalers with long-acting beta2-agonists (LABA) to reduce exacerbation rates and all-cause mortality, and to improve lung function and quality of life. The most common combinations of ICS and LABA used in combination inhalers are fluticasone and salmeterol, budesonide and formoterol and a new formulation of fluticasone in combination with vilanterol, which is now available. ICS have been associated with increased risk of pneumonia, but the magnitude of risk and how this compares with different ICS remain unclear. Recent reviews conducted to address their safety have not compared the relative safety of these two drugs when used alone or in combination with LABA.
Objectives: To assess the risk of pneumonia associated with the use of fluticasone and budesonide for COPD.
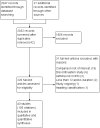
Search methods: We identified trials from the Cochrane Airways Group Specialised Register of trials (CAGR), clinicaltrials.gov, reference lists of existing systematic reviews and manufacturer websites. The most recent searches were conducted in September 2013.
Selection criteria: We included parallel-group randomised controlled trials (RCTs) of at least 12 weeks' duration. Studies were included if they compared the ICS budesonide or fluticasone versus placebo, or either ICS in combination with a LABA versus the same LABA as monotherapy for people with COPD.
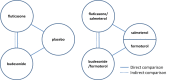
Data collection and analysis: Two review authors independently extracted study characteristics, numerical data and risk of bias information for each included study.We looked at direct comparisons of ICS versus placebo separately from comparisons of ICS/LABA versus LABA for all outcomes, and we combined these with subgroups when no important heterogeneity was noted. After assessing for transitivity, we conducted an indirect comparison to compare budesonide versus fluticasone monotherapy, but we could not do the same for the combination therapies because of systematic differences between the budesonide and fluticasone combination data sets.When appropriate, we explored the effects of ICS dose, duration of ICS therapy and baseline severity on the primary outcome. Findings of all outcomes are presented in 'Summary of findings' tables using GRADEPro.
Main results: We found 43 studies that met the inclusion criteria, and more evidence was provided for fluticasone (26 studies; n = 21,247) than for budesonide (17 studies; n = 10,150). Evidence from the budesonide studies was more inconsistent and less precise, and the studies were shorter. The populations within studies were more often male with a mean age of around 63, mean pack-years smoked over 40 and mean predicted forced expiratory volume of one second (FEV1) less than 50%.High or uneven dropout was considered a high risk of bias in almost 40% of the trials, but conclusions for the primary outcome did not change when the trials at high risk of bias were removed in a sensitivity analysis.Fluticasone increased non-fatal serious adverse pneumonia events (requiring hospital admission) (odds ratio (OR) 1.78, 95% confidence interval (CI) 1.50 to 2.12; 18 more per 1000 treated over 18 months; high quality), and no evidence suggested that this outcome was reduced by delivering it in combination with salmeterol or vilanterol (subgroup differences: I(2) = 0%, P value 0.51), or that different doses, trial duration or baseline severity significantly affected the estimate. Budesonide also increased non-fatal serious adverse pneumonia events compared with placebo, but the effect was less precise and was based on shorter trials (OR 1.62, 95% CI 1.00 to 2.62; six more per 1000 treated over nine months; moderate quality). Some of the variation in the budesonide data could be explained by a significant difference between the two commonly used doses: 640 mcg was associated with a larger effect than 320 mcg relative to placebo (subgroup differences: I(2) = 74%, P value 0.05).An indirect comparison of budesonide versus fluticasone monotherapy revealed no significant differences with respect to serious adverse events (pneumonia-related or all-cause) or mortality. The risk of any pneumonia event (i.e. less serious cases treated in the community) was higher with fluticasone than with budesonide (OR 1.86, 95% CI 1.04 to 3.34); this was the only significant difference reported between the two drugs. However, this finding should be interpreted with caution because of possible differences in the assignment of pneumonia diagnosis, and because no trials directly compared the two drugs.No significant difference in overall mortality rates was observed between either of the inhaled steroids and the control interventions (both high-quality evidence), and pneumonia-related deaths were too rare to permit conclusions to be drawn.
Authors' conclusions: Budesonide and fluticasone, delivered alone or in combination with a LABA, are associated with increased risk of serious adverse pneumonia events, but neither significantly affected mortality compared with controls. The safety concerns highlighted in this review should be balanced with recent cohort data and established randomised evidence of efficacy regarding exacerbations and quality of life. Comparison of the two drugs revealed no statistically significant difference in serious pneumonias, mortality or serious adverse events. Fluticasone was associated with higher risk of any pneumonia when compared with budesonide (i.e. less serious cases dealt with in the community), but variation in the definitions used by the respective manufacturers is a potential confounding factor in their comparison.Primary research should accurately measure pneumonia outcomes and should clarify both the definition and the method of diagnosis used, especially for new formulations such as fluticasone furoate, for which little evidence of the associated pneumonia risk is currently available. Similarly, systematic reviews and cohorts should address the reliability of assigning 'pneumonia' as an adverse event or cause of death and should determine how this affects the applicability of findings.
Conflict of interest statement
None known.
Figures





















Comment in
-
ACP Journal Club. Review: In COPD, fluticasone or budesonide increases serious pneumonia but not mortality.Ann Intern Med. 2014 Aug 19;161(4):JC8. doi: 10.7326/0003-4819-161-4-201408190-02008. Ann Intern Med. 2014. PMID: 25133387 No abstract available.
References
References to studies included in this review
Anzueto 2009 {published and unpublished data}
-
- Anzueto A, Ferguson GT, Feldman G, Chinsky K, Seibert A, Emmett A, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. Journal of Chronic Obstructive Pulmonary Disease 2009;6(5):320‐9. - PubMed
-
- Dalal AA, Blanchette CM, Petersen H, St. Charles M. Cost‐effectiveness of fluticasone propionate/salmeterol (250/50mcg) compared to salmeterol (50mcg) in patients with chronic obstructive pulmonary disease: economic evaluation of study SCO100250 [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20; San Diego. 2009:A1502 [Poster K54].
-
- GlaxoSmithKline. A Randomized, Double‐Blind, Parallel‐Group, 52‐Week Study to Compare the Effect of Fluticasone Propionate/Salmeterol DISKUS 250/50mcg BID with Salmeterol DISKUS 50mcg BID on the Annual Rate of Moderate/Severe Exacerbations in Subjects with Chronic Obstructive Pulmonary Disease (COPD). SCO100250. www.gsk‐clinicalstudyregister.com/ (accessed 25 May 2013).
-
- GlaxoSmithKline. A randomized, double‐blind, parallel‐group, 52‐week study to compare the effect of fluticasone propionate/salmeterol DISKUS 250/50mcg bid with salmeterol DISKUS 50mcg bid on the annual rate of moderate/severe exacerbations in subjects with chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com/ (accessed 8 April 2013).
Bourbeau 1998 {published data only}
Bourbeau 2007 {published data only}
Burge 2000 {published and unpublished data}
-
- Bale G, Martinez‐Camblor P, Burge PS, Soriano JB. Long‐term mortality follow‐up of the ISOLDE participants: causes of death during 13 years after trial completion. Respiratory Medicine 2008;102(10):1468‐72. - PubMed
-
- Bale GA, Burge PS, Burge C, Moore VC. The ISOLDE Study 11‐13 years on: what were the causes of death? [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco. 2007:Poster H42.
-
- Briggs AH, Lozano‐Ortega G, Spencer S, Bale G, Spencer MD, Burge PS. Estimating the cost‐effectiveness of fluticasone propionate for treating chronic obstructive pulmonary disease in the presence of missing data. Value In Health 2006;9(4):227‐35. - PubMed
-
- Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. British Medical Journal 2000;320(7245):1297‐303. - PMC - PubMed
Calverley 2003b {published and unpublished data}
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:C22 Poster 505.
-
- Calverley PM, Bonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. European Respiratory Journal 2003;22(6):912‐9. - PubMed
-
- Calverley PMA. Effect of budesonide/formoterol on severe exacerbations and lung function in moderate to severe COPD. Thorax 2002;57 Suppl III:iii44.
-
- Calverley PMA, Cseke Z, Peterson S. Budesonide/formoterol reduces the use of oral corticosteroids in the treatment of COPD [Abstract]. European Respiratory Journal 2003;22 Suppl 45:P436. - PubMed
-
- Calverley PMA, Kuna P, Olsson H. COPD exacerbations are reduced by budesonide/formoterol in a single inhaler [Abstract]. European Respiratory Journal 2003;22 Suppl 45:P1587.
Calverley 2003 TRISTAN {published and unpublished data}
-
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361(9356):449‐56. - PubMed
-
- Calverley PMA, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Safety of salmeterol/fluticasone propionate combination in the treatment of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20(Suppl 38):242s [P1572].
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Clinical improvements with salmeterol / fluticasone propionate combination in differing severities of COPD [A035] [Poster D50]. http://www.abstracts2view.com (accessed 20 April 2012).
-
- Calverley PMA, Pauwels RA, Vestbo J, Jones PW, Pride NB, Gulsvik A, et al. Salmeterol/fluticasone propionate combination for one year provides greater clinical benefit than its individual components [A98] [Poster 306]. http://www.abstracts‐on‐line.com/abstracts/ATS (accessed 20 April 2012).
-
- Hunjan MK, Chandler F. Numbers needed to treat (NNT) to avoid an exacerbation in patients with chronic obstructive pulmonary disease (COPD) using salmeterol/fluticasone propionate combination (SFC) and associated costs [Abstract]. American Thoracic Society 100th International Conference; 2004 May 21‐26; Orlando. 2004:D22 Poster 503.
Calverley 2007 TORCH {published and unpublished data}
-
- Allegra L. TORCH study: An invitation to clinical considerations. GIMT ‐ Giornale Italiano delle Malattie del Torace 2007;61(1):15‐23.
-
- Briggs AH, Glick HA, Lozano‐Ortega G, Spencer M, Calverley PM, Jones PW, et al. Is treatment with ICS and LABA cost‐effective for COPD? Multinational economic analysis of the TORCH study. European Respiratory Journal 2010;35(3):532‐9. - PubMed
-
- Briggs AH, Lozano‐Ortega G, Spencer S, Bale G, Spencer MD, Burge PS. Estimating the cost‐effectiveness of fluticasone propionate for treating chronic obstructive pulmonary disease in the presence of missing data. Value in Health 2006;9(4):227‐35. - PubMed
-
- Calverley P, Celli B, Anderson J, Ferguson G, Jenkins C, Jones P, et al. Salmeterol/fluticasone propionate combination (SFC) improves survival in COPD over three years: on‐treatment analysis from the TORCH study [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20; San Diego. 2009:A6191[Poster #219].
-
- Calverley P, Celli B, Anderson JA, Ferguson GT, Jenkins C, Jones PW, et al. The TOwards a Revolution in COPD Health (TORCH) study: salmeterol/fluticasone propionate improves survival in COPD over three years [Abstract]. Respirology 2006;11 Suppl 5:A149 [PS‐3‐8].
Calverley 2010 {published data only}
-
- Calverley PMA, Kuna P, Monso E, Costantini M, Petruzzelli Sd, Sergio F, et al. Beclomethasone/formoterol in the management of COPD: a randomised controlled trial. Respiratory Medicine 2010;104:1858‐68. - PubMed
Choudhury 2005 {published data only}
-
- Choudhury AB, Dawson CM, Kilvington HE, Eldridge HE, James WY, Wedzicha JA, et al. Withdrawal of inhaled corticosteroids in people with chronic obstructive pulmonary disease (COPD) in primary care ‐ a randomised controlled trial [Abstract]. European Respiratory Journal 2005;26 Suppl 49:Abstract No. 1328.
Dal Negro 2003 {published data only}
-
- Dal Negro R, Micheletto C, Trevsian F, Tognella S. Salmeterol and fluticasone 50ug/250ug BiD versus salmeterol 50ug bid and versus placebo in the long term treatment of COPD. American Thoracic Society 98th International Conference; May 17‐22; Georgia. 2002.
-
- Dal Negro RW, Pomari C, Tognella S, Micheletto C. Salmeterol & fluticasone 50 mcg/250 mcg bid in combination provides a better long‐term control than salmeterol 50 microg bid alone and placebo in COPD patients already treated with theophylline. Pulmonary Pharmacology and Therapeutics 2003;16(4):241‐6. - PubMed
Dransfield 2013 {published data only}
-
- Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, et al. Once‐daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbation of COPD: two replicate double‐blind, parallel‐group, randomised controlled trials. The Lancet: Respiratory Medicine 2013;1(3):210‐23. - PubMed
Ferguson 2008 {published data only}
-
- Dalal AA, Blanchette CM, Petersen H, Manavi K, St. Charles M. Cost‐effectiveness of fluticasone propionate/salmeterol (250/50 mcg) compared to salmeterol (50 mcg) in patients with COPD: economic evaluation of a randomized, double‐blind, parallel‐group, multi‐centre trial (Study SCO40043) [Abstract]. Chest 2008;134(4):106003s.
-
- Ferguson G, Anzueto A, Fei R, Emmett A, Crater G, Knobil K, et al. A randomized, double‐blind trial comparing the effect of fluticasone/salmeterol 250/50 to salmeterol on COPD exacerbations in patients with COPD. Chest 2007;132(4):530b‐531.
-
- Ferguson GT, Anzueto A, Fei R, Emmett A, Knobil K, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50 mcg) or salmeterol (50 mcg) on COPD exacerbations. Respiratory Medicine 2008;102:1099‐108. - PubMed
-
- SCO40043. A randomized, double‐blind, parallel‐group, 52‐week study to compare the effect of fluticasone propionate/salmeterol DISKUS® 250/50mcg bid with salmeterol DISKUS® 50mcg bid on the annual rate of moderate/severe exacerbations in subjects with chronic obstructive pulmonary disease (COPD). http://ctr.gsk.co.uk (accessed 8 April 2008).
Fukuchi 2013 {published data only}
-
- Fukuchi Y, Samoro R, Fassakhov R, Taniguchi H, Ekelund J, Carlsson LG, et al. Budesonide/formoterol via Turbuhaler® versus formoterol via Turbuhaler® in patients with moderate to severe chronic obstructive pulmonary disease: Phase III multinational study results. Respirology 2013; Vol. 18:866‐73. - PubMed
-
- Ichinose M, Samoro R, Fassakhov R, Oguri M, Ekelund J, Carlsson LG, et al. Budesonide/formoterol vs formoterol, both via Turbuhaler®, in patients with moderate to severe COPD: Phase III study results [Abstract]. European Respiratory Society Annual Congress; 2012 Sep 1‐5; Vienna. 2012; Vol. 40, issue Suppl 56:374s [P2108].
GSK FCO30002 2005 {unpublished data only}
-
- FCO30002. A multi‐centre, randomised, placebo‐controlled, double‐blind comparison with 3 parallel groups to investigate the efficacy and safety of inhaled glucocorticoid fluticasone (500 μg bd via Diskus™) vs. oral glucocorticoid therapy vs. placebo in subjects with chronic obstructive airway disease (COPD) under therapy with Salmeterol (50 μg bd). GlaxoSmithKline Clinical Trial Register (accessed 15 April 2012).
GSK FLTA3025 2005 {unpublished data only}
-
- FLTA3025. A randomized, double‐blind, parallel‐group, comparative trial of inhaled fluticasone propionate 250mcg BID, 500mcg BID, and placebo BID via the DISKUS in subjects with chronic obstructive pulmonary disease (COPD). GlaxoSmithKline Clinical Trial Register (accessed 18 April 2012).
GSK SCO100470 2006 {unpublished data only}
-
- SCO100470. A multi‐centre, randomised, double‐blind, parallel group, 24‐week study to compare the effect of the salmeterol/fluticasone propionate combination product 50/250mcg, with salmeterol 50mcg both delivered twice daily via the DISKUS/ACCUHALER inhaler on lung function and dyspnoea in subjects with Chronic Obstructive Pulmonary Disease (COPD). http:ctr.gsk.co.uk (accessed 20 April 2012).
GSK SCO104925 2008 {unpublished data only}
-
- Evaluation of Novel Endpoints in Subjects with Chronic Obstructive Pulmonary Disease(COPD) in a Randomized, Double‐Blind, Placebo‐Controlled Study of Treatment with FluticasonePropionate/Salmeterol 500/50mcg combination and its individual components, FluticasonePropionate 500mcg and Salmeterol 50mcg. http://www.gsk‐clinicalstudyregister.com/ 2008 (accessed 19 April 2012).
-
- SCO104925. Evaluation of Novel Endpoints in Subjects with Chronic Obstructive Pulmonary Disease(COPD) in a Randomized, Double‐Blind, Placebo‐Controlled Study of Treatment with FluticasonePropionate/Salmeterol 500/50mcg combination and its individual components, FluticasonePropionate 500mcg and Salmeterol 50mcg. http:ctr.gsk.co.uk (accessed 19 April 2012).
GSK SCO30002 2005 {unpublished data only}
-
- SCO30002. A multi‐centre, randomised, double‐blind, parallel group, placebo‐controlled study to compare the efficacy and safety of inhaled salmeterol/fluticasone propionate combination product 25/250 µg two puffs bd and fluticasone propionate 250µg two puffs bd alone, all administered via metered dose inhalers (MDI), in the treatment of subjects with chronic obstructive pulmonary disease (COPD) for 52 weeks. GlaxoSmithKline Clinical Trial Register (accessed 21 April 2012).
GSK SCO40041 2008 {unpublished data only}
-
- NCT00355342. A Randomized, Double‐Blind, Parallel‐Group Clinical Trial Evaluating the Effect of the Fluticasone Propionate/Salmeterol Combination Product 250/50mcg BID Via DISKUS Versus Salmeterol 50mcg BID Via DISKUS on Bone Mineral Density in Subjects With Chronic Obstructive Pulmonary Disease (COPD). http://clinicaltrials.gov/show/NCT00355342 (accessed 19 April 2012).
Hanania 2003 {published data only}
-
- Hanania NA, Darken P, Horstman D, Reisner C, Lee B, Davis S, et al. The efficacy and safety of fluticasone propionate (250 mcg)/salmeterol (50 mcg) combined in the Diskus inhaler for the treatment of COPD. Chest 2003;124(3):834‐43. - PubMed
-
- Hanania NA, Knobil K, Watkins M, Wire P, Yates J, Darken P. Salmeterol and fluticasone propionate therapy administered by a single Diskus in patients with COPD. Chest 2002:S129.
-
- Hanania NA, Ramsdell J, Payne K, Davis S, Horstman D, Lee B, et al. Improvements in airflow and dyspnoea in COPD patients following 24 weeks treatment with salmeterol 50mcg and fluticasone propionate 250mcg alone or in combination via the Diskus. American Journal of Respiratory and Critical Care Medicine 2001;163 Suppl 5:A279.
-
- Horstman D, Darken P, Davis S, Lee B. Improvements in FEV1 and symptoms in poorly reversible COPD patients following treatment with salmeterol 50mcg/fluticasone propionate 250mcg combination [Abstract]. European Respiratory Journal 2003;22 Suppl 45:P434.
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD) [Abstract]. National COPD Conference; 2003 Nov 14‐15; Arlington. 2003:1081.
Hattotuwa 2002 {published data only}
-
- Hattotuwa KL, Gizycki MJ, Ansari TW, Jeffery PK, Barnes NC. The effects of inhaled fluticasone on airway inflammation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2002;165(12):1592‐9. - PubMed
Kardos 2007 {published and unpublished data}
-
- Kardos P, Wencker M. Combination therapy with salmeterol and fluticasone propionate (SFC) is more effective than salmeterol (SAL) alone in reducing exacerbations of COPD. European Respiratory Journal 2005;26 Suppl 49:Abstract 1944.
-
- Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2007;175(2):144‐9. - PubMed
-
- SCO30006. A randomised, double‐blind, parallel‐group study to investigate the protective effect of the combination of fluticasone and salmeterol (500/50 µg bid via the DISKUS) compared with salmeterol (50 µg bid via the DISKUS) on the incidence of moderate to severe exacerbations in patients with severe chronic obstructive pulmonary disease (COPD) (GOLD III/IV). http:ctr.gsk.co.uk (accessed 21 April 2012).
-
- Vogelmeier C. Combination therapy with salmeterol and fluticasone propionate (SFC) improves quality of life (QoL) more than salmeterol (SAL) alone in COPD. 42nd Nordic Lung Conference; 2005 Jun 9‐11; Trondheim. 2005; Vol. 13 Suppl 22.
-
- Vogelmeier CF, Wencker M, Glaab TH, Kardos P. Number needed to treat (NNT) to reduce exacerbations in severe COPD comparing salmeterol/fluticasone propionate (SFC) with salmeterol (SAL) treatment.. American Thoracic Society International Conference; May 19‐21; San Diego. 2006:A110.
Kerwin 2013 {published data only}
-
- Kerwin EM, Scott‐Wilson C, Sanford L, Rennard S, Agusti A, Barnes N, et al. A randomised trial of fluticasone furoate/vilanterol (50/25 mug; 100/25 mug) on lung function in COPD. Respiratory Medicine 2013; Vol. 107, issue 4:560‐9. [0954‐6111] - PubMed
-
- Kerwin EM, Scott‐Wilson C, Sanford L, Rennard SI, Agusti A, Barnes N, et al. Lung function effects and safety of fluticasone furoate (FF)/vilanterol (VI) in patients with COPD: Low‐mid dose assessment [Abstract]. European Respiratory Society Annual Congress; Sep 1‐5; Vienna. 2012; Vol. 40, issue Suppl 56:545s [3082].
Lapperre 2009 {published and unpublished data}
-
- Lapperre TS, Snoeck‐Stroband JB, Gosman MM, Jansen DF, Schadewijk A, Thiadens HA, et al. Effect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2009;151(8):517‐27. - PubMed
Laptseva 2002 {published data only}
-
- Laptseva IM, Laptseva EA, Borshchevsky VV, Gurevich G, Kalechits O. Inhaled budesonide in the management of chronic obstructive pulmonary disease. European Respiratory Journal 2002;20 Suppl 38:244s.
Mahler 2002 {published and unpublished data}
-
- Mahler DA, Darken P, Brown CP, Knobil K. Predicting lung function responses to combination therapy in chronic obstructive pulmonary disease (COPD). http://www.abstracts2view.com (accessed 15 April 2012).
-
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2002;166(8):1084‐91. - PubMed
-
- SFCA3006. A randomized, double‐blind, placebo‐controlled, parallel‐group trial evaluating the safety and efficacy of the DISKUS formulations of salmeterol (SAL) 50mcg BID and fluticasone propionate (FP) 500mcg BID individually and in combination as salmeterol 50mcg/fluticasone propionate 500mcg BID (SFC 50/500) compared to placebo in COPD subjects. http://ctr.gsk.co.uk (accessed 17 April 2012).
-
- Spencer M, Wire P, Lee B, Chang CN, Darken P, Horstman D. Patients with COPD using salmeterol/fluticasone propionate combination therapy experience improved quality of life. European Respiratory Journal 2003;22 Suppl 45:51.
-
- Spencer MD, Anderson JA. Salmeterol/fluticasone combination produces clinically important benefits in dyspnoea and fatigue [Abstract]. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego. 2005:B93 [Poster 308].
Martinez 2013 {published data only}
-
- Martinez F, Boscia J, Feldman G, Scott‐Wilson C, Kilbride S, Fabbri L, et al. Lung function effects and safety of fluticasone furoate (FF)/vilanterol (VI) in patients with COPD: mid‐high dose assessment [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 1‐5. 2012; Vol. 40, issue Suppl 56:527s [P2887].
-
- Martinez FJ, Boscia J, Feldman G, Scott‐Wilson C, Kilbride S, Fabbri L, et al. Fluticasone furoate/vilanterol (100/25; 200/25 mug) improves lung function in COPD: a randomised trial. Respiratory Medicine 2013;107(4):550‐9. [0954‐6111] - PubMed
Mirici 2001 {published data only}
-
- Mirici A, Bektas Y, Ozbakis G, Erman Z. Effect of inhaled corticosteroids on respiratory function tests and airway inflammation in stable chronic obstructive pulmonary disease. Clinical Drug Investigation 2001;21(12):835‐42.
Ozol 2005 {published data only}
-
- Ozol D, Aysan T, Solak ZA, Mogulkoc N, Veral A, Sebik F. The effect of inhaled corticosteroids on bronchoalveolar lavage cells and IL‐8 levels in stable COPD patients. Respiratory Medicine 2005;99(12):1494‐500. - PubMed
Paggiaro 1998 {published data only}
-
- FLIT97. A multi‐centre, randomised, double‐blind, parallel group study of the efficacy and safety of inhaled fluticasone propionate 1000 µg daily with placebo in chronic obstructive pulmonary disease. http://ctr.gsk.co.uk (accessed 20 April 2012).
-
- Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo‐controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study Group. Lancet 1998;351(9105):773‐80. - PubMed
Pauwels 1999 {published data only}
-
- Johnell O, Pauwels R, Löfdahl C‐G, Laitinen LA, Postma DS, Pride NB, et al. Bone mineral density in patients with chronic obstructive pulmonary disease treated with budesonide Turbuhaler. European Respiratory Journal 2002;19(6):1058‐63. - PubMed
-
- Lofdahl CG, Postma DS, Laitinen LA, Ohlsson SV, Pauwels RA, Pride NB. The European Respiratory Society study on chronic obstructive pulmonary disease (EUROSCOP): recruitment methods and strategies. Respiratory Medicine 1998;92(3):467‐72. - PubMed
-
- Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, et al. Long‐term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. New England Journal of Medicine 1999;340(25):1948‐53. - PubMed
-
- Pauwels RA, Lofdahl CG, Pride NB, Postma DS, Laitinen LA, Ohlsson SV. European Respiratory Society study on chronic obstructive pulmonary disease (EUROSCOP): hypothesis and design. European Respiratory Journal 1992;5(10):1254‐61. - PubMed
Renkema 1996 {published data only}
-
- Renkema TE, Schouten JP, Koeter GH, Postma DS. Effects of long‐term treatment with corticosteroids in COPD. Chest 1996;109(5):1156‐62. - PubMed
Rennard 2009 {published data only}
-
- Rennard SI, Tashkin DP, McElhattan J, Goldman M, Ramachandran S, Martin UJ, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered‐dose inhaler in patients with chronic obstructive pulmonary disease. Results from a 1‐year randomized controlled clinical trial. Drugs 2009;69(5):549‐65. - PMC - PubMed
Schermer 2009 {published data only}
-
- Schermer T, Chavannes N, Dekhuijzen R, Wouters E, Muris J, Akkermans R, et al. Fluticasone and N‐acetylcysteine in primary care patients with COPD or chronic bronchitis. Respiratory Medicine 2009;103(4):542‐51. - PubMed
Senderovitz 1999 {published data only}
-
- Senderovitz T, Vestbo J, Frandsen J, Maltbaek N, Norgaard M, Nielsen C, et al. Steroid reversibility test followed by inhaled budesonide or placebo in outpatients with stable chronic obstructive pulmonary disease. Respiratory Medicine 1999;93(10):715‐8. - PubMed
Shaker 2009 {published and unpublished data}
-
- Shaker SB, Dirksen A, Ulrik CS, Hestad M, Stavngaard T, Laursen LC, et al. The effect of inhaled corticosteroids on the development of emphysema in smokers assessed by annual computed tomography. COPD: Journal of Chronic Obstructive Pulmonary Disease 2009;6(2):104‐11. - PubMed
-
- Shaker SB, Stavngaard T, Laursen LC, Stoel BC, Dirksen A. Rapid fall in lung density following smoking cessation in COPD. COPD: Journal of Chronic Obstructive Pulmonary Disease 2011;8(1):2‐7. - PubMed
Sharafkhaneh 2012 {published data only}
-
- Sharafkhaneh A, Southard JG, Goldman M, Uryniak T, Martin UJ. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double‐blind, randomized study. Respiratory Medicine 2012;106(2):257‐68. - PubMed
-
- Sharafkhaneh A, Uryniak T, Martin U. Long‐term effects of budesonide/formoterol pressurized metered‐dose inhaler on Chronic Obstructive Pulmonary Disease (COPD) symptoms and health status in patients with COPD [Abstract]. Chest 2011;140(4):528A.
Szafranski 2003 {published data only}
-
- Anderson P. Budesonide/formoterol in a single inhaler (Symbicort) provides early and sustained improvement in lung function in moderate to severe COPD. Thorax 2002;57 Suppl 3:iii43.
-
- Borgstrom L, Asking L, Olsson H, Peterson S. Lack of interaction between disease severity and therapeutic response with budesonide/formoterol in a single inhaler [Abstract].. American Thoracic Society 100th International Conference; May 21‐26; Orlando. 2004:C22 [Poster 505].
-
- Calverley P, Pauwels Dagger R, Löfdahl CG, Svensson K, Higenbottam T, Carlsson LG, et al. Relationship between respiratory symptoms and medical treatment in exacerbations of COPD. European Respiratory Journal 2005;26(3):406‐13. - PubMed
-
- Calverley PMA. Effect of budesonide/formoterol on severe exacerbations and lung function in moderate to severe COPD. Thorax 2002;BTS Winter Meeting 2002:S145.
-
- Calverley PMA, Szafranski W, Andersson A. Budesonide/formoterol is a well‐tolerated long term maintenance therapy for COPD. European Respiratory Journal 2005;26 Suppl 49:Poster 1917.
Tashkin 2008 SHINE {published and unpublished data}
-
- AstraZeneca. A 6‐month double‐blind, double‐dummy, randomized, parallel group, multicenter efficacy & safety study of SYMBICORT® pMDI 2 x 160/4.5 μg & 80/4.5 μg bid compared to formoterol TBH, budesonide pMDI (& the combination) & placebo in COPD Patients (SHINE) (study number D589900002). www.astrazenecaclinicaltrials.com (accessed 18 April 2012).
-
- Bleecker ER, Meyers DA, Bailey WC, Sims AM, Bujac SR, Goldman M, et al. Effect of ß2‐Adrenergic Receptor Gene Polymorphism Gly16Arg on Response to Budesonide/Formoterol Pressurized Metered‐Dose Inhaler in Chronic Obstructive Pulmonary Disease [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183:A4086.
-
- Tashkin DP, Rennard SI, Martin P, Ramachandran S, Martin UJ, Silkoff PE. Efficacy and safety of budesonide and formoterol in one pressurized metered‐dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6‐month randomized clinical trial. Drugs 2008;68(14):1975‐2000. - PubMed
van Grunsven 2003 {published data only}
-
- Grunsven P, Schermer T, Akkermans R, Albers M, Boom G, Schayck O, et al. Short‐ and long‐term efficacy of fluticasone propionate in subjects with early signs and symptoms of chronic obstructive pulmonary disease. Results of the DIMCA study. Respiratory Medicine 2003;97(12):1303‐12. - PubMed
Verhoeven 2002 {published and unpublished data}
Vestbo 1999 {published data only}
-
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long‐term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 1999;353(9167):1819‐23. [MEDLINE: ] - PubMed
Yildiz 2004 {published data only}
-
- Yildiz F, Basyigit I, Yildirim E, Boyaci H, Ilgazli A. Does addition of inhaled steroids to combined bronchodilator therapy affect health status in patients with COPD?. Respirology 2004;9(3):352‐5. - PubMed
References to studies excluded from this review
GSK FLIP63 2005 {published data only}
-
- GlaxoSmithKline F. A single‐centre, double‐blind study to investigate the effects of inhaled fluticasone propionate (FP) 1500mcgdaily on inflammatory processes in the lungs of patients with chronic obstructive airways disease [FLIP63]. www.gsk‐clinicalstudyregister.com (accessed 20 April 2012).
GSK SAM30022 2005 {published data only}
-
- GSK SAM30022. A phase IV, multi‐centre, double‐blind, double‐dummy, parallel group, randomised study comparing Seretide (25/50 2 puffs bd) via the Evohaler (MDI‐HFA) with Beclometasone dipropionate (200mcg 2 puffs bd) via the MDI‐CFC in adolescents and adults experiencing moderate symptoms of reversible airways obstruction. www.gsk‐clinicalstudyregister.com (accessed 20 April 2012).
GSK SAM40004 2006 {published data only}
-
- GSK SAM40004. A multi‐centre, randomised, double‐blind, placebo‐controlled parallel group study to compare the effect on airway inflammation and remodelling of treatment with salmeterol/fluticasone propionate combination product (50/100μg strength) bd via the Accuhaler inhaler, or fluticasone propionate 100μg bd via the Accuhaler inhaler or placebo via the Accuhaler inhaler for 16 weeks, followed by double‐blind treatment for 52 weeks with the salmeterol/fluticasone propionate combination product (50/100μg strength) bd via the Accuhaler inhaler or fluticasone propionate 100μg bd via the Accuhaler inhaler, in adults with reversible airways obstruction (SIRIAS ‐ Seretide in Inflammation and Remodelling In Asthma Study). www.gsk‐clinicalstudyregister.com (accessed 20 April 2012).
GSK SAS40015 2007 {published data only}
-
- GSK SAS40015. A multi‐centre, randomised, double‐blind, double‐dummy, parallel‐group, 12‐week, active control comparison of the salmeterol/fluticasone propionate combination product (50/100 mcg strength) bd via the DISKUS/ACCUHALER inhaler with fluticasone propionate (100 mcg strength) bd via the DISKUS/ACCUHALER inhaler plus oral montelukast 10 mg od in adolescents and adults with reversible airways obstruction [SAS40015]. www.gsk‐clinicalstudyregister.com (accessed 20 April 2012).
GSK SCO100540 2006 {published data only}
-
- GSK SCO100540. A multi‐centre, randomised, double‐blind, parallel group study to investigate the efficacy and safety of the Salmeterol/fluticasone propionate combination at a strength of 50/500µg BD, compared with placebo via Accuhaler, added to usual chronic obstructive pulmonary disease (COPD) therapy, in subjects with COPD for 24 weeks [SCO100540]. www.gsk‐clinicalstudyregister.com (accessed 20 April 2012).
GSK SCO100646 2008 {published data only}
-
- GSK SCO100646. Clinical Evaluation of SFC for Chronic Obstructive Pulmonary Disease (Chronic Bronchitis, Emphysema) ‐Assessment of the Effect of Addition of Fluticasone Propionate to Salmeterol Xinafoate 50 mcg after Switching under Double‐blinded Conditions to SFC 50/250 mcg in Chronic Obstructive Pulmonary Disease. Study number SCO100646. www.gsk‐clinicalstudyregister.com (accessed 20 April 2012).
GSK SCO30005 2006 {published data only}
-
- GSK SCO30005. A 13‐week, double‐blind, parallel‐group, multi‐centre study to compare the bronchial anti‐inflammatory activity of the combination of salmeterol/ fluticasone propionate (SERETIDE™/ADVAIR™/VIANI™) 50/500 mcg twice daily compared with placebo twice daily in patients with Chronic Obstructive Pulmonary Disease [SCO30005]. www.gsk‐clinicalstudyregister.com (accessed 20 April 2012).
GSK SCO40030 2005 {published data only}
-
- GSK SCO40030. A Randomized, Double‐Blind, Placebo‐Controlled, Parallel Group Clinical Trial Evaluating the Effect of the Fluticasone Propionate/Salmeterol Combination Product 250/50mcg BID via DISKUS and Salmeterol 50mcg BID via DISKUS on Lung Hyperinflation in Subjects with Chronic Obstructive Pulmonary Disease (COPD). [SCO40030]. www.gsk‐clinicalstudyregister.com (accessed 20 April 2012).
GSK SCO40034 2009 {published data only}
-
- GSK SCO40034. A multi‐centre, randomised, double‐blind, double dummy, parallel group 12‐week exploratory study to compare the effect of the fluticasone/salmeterol propionate combination product (SERETIDE™) 50/500mcg bd via the DISKUS™/ACCUHALER™ inhaler with tiotropium bromide 18 mcg od via the Handihaler inhalation device on efficacy and safety in patients with Chronic Obstructive Pulmonary Disease (COPD). www.gsk‐clinicalstudyregister.com (accessed 20 April 2012).
GSK SCO40036 2009 {published data only}
-
- GSK SCO40036. Multicentre, Randomised, Double‐Blind, Double Dummy, Parallel Group, 104‐week Study to Compare the Effect of the Salmeterol/Fluticasone Propionate Combination Product (SERETIDE*) 50/500mcg Delivered Twice Daily via the DISKUS*/ACCUHALER* Inhaler with Tiotropium Bromide 18 mcg Delivered Once Daily via the HandiHaler Inhalation Device on the Rate of Health Care Utilisation Exacerbations in Subjects with Severe Chronic Obstructive Pulmonary Disease (COPD). www.gsk‐clinicalstudyregister.com (accessed 20 April 2012).
-
- GSK SCO40036. Multicentre, randomised, double‐blind, double‐dummy, parallel group, 104‐week study to compare the effect of the salmeterol/fluticasone propionate combination product (SERETIDE*) 50/500mcg delivered twice daily via the DISKUS*/ACCUHALER* inhaler with tiotropium bromide 18 mcg delivered once daily via the HandiHaler inhalation device on the rate of health care utilisation exacerbations in subjects with severe chronic obstructive pulmonary disease (COPD). www.gsk‐clinicalstudyregister.com (accessed 8 April 2012).
GSK SFCB3019 2004 {published data only}
-
- GSK SFCB3019. A multi‐centre randomized, double‐blind, double‐dummy, parallel‐group comparison of the salmeterol/fluticasone propionate combination product (50/500mcg strength) BD via one DISKUS/Accuhaler inhaler with salmeterol 50mcg BD via one DISKUS/Accuhaler and fluticasone propionate 500mcg BD via another DISKUS/Accuhaler and with fluticasone propionate 500mcg BD via one DISKUS/Accuhaler in adolescents and adults with reversible airways obstruction. www.gsk‐clinicalstudyregister.com (accessed 20 April 2012).
Lung Health Study 2000 {published data only}
-
- Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. New England Journal of Medicine 2000;343(26):1902‐9. - PubMed
van der Valk 2002 {published data only}
-
- Monninkhof E, Valk P, Palen J, Zielhuis G, Hervaarden C, Herwaarden C. Effect of discontinuation of inhaled steroids on health‐related quality of life in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2002;165 Suppl 8:A226. - PubMed
-
- Valk P, Monninkhof E, Palen J, Zielhuis G, Herwaarden C, Phalen J. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. American Journal of Respiratory and Critical Care Medicine 2002;166(1073‐449X):1358‐63. - PubMed
-
- Valk P, Monninkhof EM, Palen JAM, Zielhuis GA, Herwaarden CLA. Effect of discontinuation of inhaled fluticasone propionate on exacerbations in patients with COPD. European Respiratory Journal 2001;18 Suppl 33:183s.
Weir 1999 {published data only}
-
- Weir DC, Bale GA, Bright P, Sherwood Burge P. A double‐blind placebo‐controlled study of the effect of inhaled beclomethasone dipropionate for 2 years in patients with nonasthmatic chronic obstructive pulmonary disease. Clinical and Experimental Allergy 1999;29(Suppl 2):125‐8. - PubMed
Wouters 2005 {published data only}
-
- Wouters EF. COPD and Seretide: a multi‐centre intervention and characterisation (COSMIC) study: a rationale and baseline characteristics [abstract]. European Respiratory Society Annual Congress; Sep 14‐18; Stockholm. 2002:P1571.
-
- Wouters EF, Postma DS, Fokkens B, Hop WC, Prins J, Kuipers AFTCC, et al. [Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial]. [Portuguese]. Revista Portuguesa de Pneumologia 2005;11(6):587‐9. - PubMed
References to studies awaiting assessment
Ohar 2013 {published data only}
-
- Ohar JA, Crater G, Emmett A, Ferro T, Morris A, Raphiou I, et al. Effects of Fluticasone Propionate/Salmeterol Combination 250/50mcg BID (ADVAIR DISKUS™) vs. Salmeterol 50mcg BID (SEREVENT DISKUS™) on Chronic Obstructive Pulmonary Disease (COPD) Exacerbation Rate, Following Acute Exacerbation or Hospitalization. American Journal of Respiratory and Critical Care Medicine. 2013; Vol. 187(Meeting Abstracts):A2439.
References to ongoing studies
Vestbo 2013 {published data only}
-
- Vestbo J, Anderson J, Brook RD, Calverley PMA, Celli BR, Crim C, et al. The study to understand mortality and morbidity in COPD (SUMMIT) study protocol. European Respiratory Journal 2013;41(5):1017‐22. [0903‐1936] - PubMed
Additional references
Agarwal 2010
-
- Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Inhaled corticosteroids vs placebo for preventing COPD exacerbations. Chest 2010;137(2):318‐25. - PubMed
Agusti 2013
-
- Agusti A, Teresa L, Backer W, Zvarich MT, Locantore N, Barnes N, et al. A comparison of the efficacy and safety of once‐daily fluticasone furoate/vilanterol with twice‐daily fluticasone propionate/salmeterol in moderate to very severe COPD. The European Respiratory Journal. 2013/10/12 2013 Oct 10 [Epub ahead of print]. [1399‐3003: (Electronic)]
ATS/ERS 2004
-
- American Thoracic Society / European Respiratory Society Task Force. Standards for the diagnosis and management of patients with COPD [Internet]. Version 1.2. http://www.thoracic.org/go/copd. New York: American Thoracic Society, (accessed 4 May 2012). [URL: http://www.thoracic.org/go/copd]
Blais 2010
-
- Blais L, Forget A, Ramachandran S. Relative effectiveness of budesonide/formoterol and fluticasone propionate/salmeterol in a 1‐year, population‐based, matched cohort study of patients with chronic obstructive pulmonary disease (COPD): effect on COPD‐related exacerbations, emergency department visits and hospitalizations, medication utilization, and treatment adherence. Clinical Therapeutics 2010;32(7):1320‐8. - PubMed
BTS 2009
-
- British Thoracic Society, Community Acquired Pneumonia in Adults Guideline Group. Guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009;64(Suppl III):iii1–iii55. - PubMed
Bucher 1997
-
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997; Vol. 50, issue 6:683‐91. [0895‐4356: (Print)] - PubMed
Cates 2013
-
- Cates C. Inhaled corticosteroids in COPD: quantifying risks and benefits. Thorax 2013;68(6):499‐500. - PubMed
Cipriani 2013
-
- Cipriani A, Higgins JPT, Geddes JR, Salanti GS. Conceptual and technical challenges in network meta‐analysis. Annals of Internal Medicine 2013;159(2):130‐7. - PubMed
Drummond 2008
Effing 2007
Ernst 2007
-
- Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. American Journal of Respiratory and Critical Care Medicine 2007;176:162‐6. - PubMed
Glenny 2005
-
- Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D'Amico R, et al. Indirect comparisons of competing interventions. Health Technology Assessment 2005;9(26):1‐134, iii‐iv. [1366‐5278: (Print)] - PubMed
GOLD 2013
-
- GOLD. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013. http://www.goldcopd.com (accessed 22 November 13).
Halpin 2011
-
- Halpin DM, Gray J, Edwards SJ, Morais J, Singh D. Budesonide/formoterol vs. salmeterol/fluticasone in COPD: a systematic review and adjusted indirect comparison of pneumonia in randomised controlled trials. International Journal of Clinical Practice 2011;65(7):764‐74. [1742‐1241: (Electronic)] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. www.cochrane‐handbook.org.
Janson 2013 [PATHOS]
-
- Janson C, Larsson K, Lisspers KH, Stallberg B, Stratelis G, Goike H, et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting beta2 agonist: observational matched cohort study (PATHOS). BMJ. 2013/05/31 2013; Vol. 346:f3306. [1756‐1833: (Electronic)] - PMC - PubMed
Johnson 1998
-
- Johnson M. Development of fluticasone propionate and comparison with other inhaled corticosteroids. Journal of Allergy and Clinical Immunology 1998;101(4):S434‐9. - PubMed
Lacasse 2006
Marzoratti 2013
-
- Marzoratti L, Iannella HA, Waterer GW. Inhaled corticosteroids and the increased risk of pneumonia. Therapeutic Advances in Respiratory Disease. 2013/03/01 2013; Vol. 7, issue 4:225‐34. [1753‐4666: (Electronic)] - PubMed
Nannini 2012
-
- Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long‐acting beta2‐agonist in one inhaler versus long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD006829.pub2] - DOI
Nannini 2013
NICE 2011
-
- National Institute for Health and Clinical Excellence. Chronic obstructive pulmonary disease, costing report, implementing NICE guidance. http://guidance.nice.org.uk/CG101/CostingReport/pdf/English (accessed 4 February 2012). [URL: http://guidance.nice.org.uk/CG101/CostingReport/pdf/English]
Partridge 2009
-
- Partridge MR, Schuermann W, Beckman O, Persson T, Polanowski T. Effect on lung function and morning activities of budesonide/formoterol versus salmeterol/fluticasone in patients with COPD. Therapeutic Advances in Respiratory Disease 2009;3(4):147‐57. - PubMed
Puhan 2011
Roberts 2011
-
- Roberts M, Mapel D, Petersen H, Blanchette C, Ramachandran S. Comparative effectiveness of budesonide/formoterol and fluticasone/salmeterol for COPD management. Journal of Medical Economics 2011;14(6):769‐76. - PubMed
Rossios 2011
-
- Rossios C, To Y, To M, Ito M, Barnes PJ, Adcock IM, Johnson M, Ito K. Long‐acting fluticasone furoate has a superior pharmacological profile to fluticasone propionate in human respiratory cells. European Journal of Pharmacology 2011;670(1):244‐51. - PubMed
Ruiz 2011
-
- Ruiz Garcia V, Compte Torrero L. [Risk of pneumonia with inhaled corticosteroids for stable chronic obstructive pulmonary disease patients]. Medicina Clinica. 2011/03/15 2011; Vol. 137, issue 7:302‐4. [0025‐7753: (Print)] - PubMed
Sin 2009
-
- Sin DD, Tashkin D, Zhang X, Radner F, Sjobring U, Thoren A, et al. Budesonide and the risk of pneumonia: a meta‐analysis of individual patient data. Lancet 2009;374(9691):712‐9. [1474‐547X: (Electronic)] - PubMed
Singanayagam 2010
-
- Singanayagam A, Chalmers J, Hill A. Inhaled corticosteroids and risk of pneumonia: evidence for and against the proposed association. Quarterly Journal of Medicine 2010;103:379‐85. - PubMed
Singh 2009
-
- Singh S, Amin AV, Loke YK. Long‐term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta‐analysis. Archives of Internal Medicine 2009;169(3):219‐29. [1538‐3679: (Electronic)] - PubMed
Song 2008
-
- Song F, Harvey I, Lilford R. Adjusted indirect comparison may be less biased than direct comparison for evaluating new pharmaceutical interventions. Journal of Clinical Epidemiology 2008;61(5):455‐63. [0895‐4356: (Print)] - PubMed
Suissa 2013
Van der Meer 2001
WHO 2012
-
- World Health Organization. Chronic respiratory diseases. http://www.who.int/respiratory/en/ (accessed 7 February 2012).
Yang 2009
Yang 2012
Yawn 2013
-
- Yawn BP, Li Y, Tian H, Zhang J, Arcona S, Kahler KH. Inhaled corticosteroid use in patients with chronic obstructive pulmonary disease and the risk of pneumonia: a retrospective claims data analysis. International Journal of Chronic Obstructive Pulmonary Disease. 2013/07/10 2013; Vol. 8:295‐304. [1178‐2005: (Electronic)] - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

