Incidence and time course of everolimus-related adverse events in postmenopausal women with hormone receptor-positive advanced breast cancer: insights from BOLERO-2
- PMID: 24615500
- PMCID: PMC3969554
- DOI: 10.1093/annonc/mdu009
Incidence and time course of everolimus-related adverse events in postmenopausal women with hormone receptor-positive advanced breast cancer: insights from BOLERO-2
Abstract
Background: In the BOLERO-2 trial, everolimus (EVE), an inhibitor of mammalian target of rapamycin, demonstrated significant clinical benefit with an acceptable safety profile when administered with exemestane (EXE) in postmenopausal women with hormone receptor-positive (HR(+)) advanced breast cancer. We report on the incidence, time course, severity, and resolution of treatment-emergent adverse events (AEs) as well as incidence of dose modifications during the extended follow-up of this study.
Patients and methods: Patients were randomized (2:1) to receive EVE 10 mg/day or placebo (PBO), with open-label EXE 25 mg/day (n = 724). The primary end point was progression-free survival. Secondary end points included overall survival, objective response rate, and safety. Safety evaluations included recording of AEs, laboratory values, dose interruptions/adjustments, and study drug discontinuations.
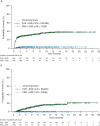
Results: The safety population comprised 720 patients (EVE + EXE, 482; PBO + EXE, 238). The median follow-up was 18 months. Class-effect toxicities, including stomatitis, pneumonitis, and hyperglycemia, were generally of mild or moderate severity and occurred relatively early after treatment initiation (except pneumonitis); incidence tapered off thereafter. EVE dose reduction and interruption (360 and 705 events, respectively) required for AE management were independent of patient age. The median duration of dose interruption was 7 days. Discontinuation of both study drugs because of AEs was higher with EVE + EXE (9%) versus PBO + EXE (3%).
Conclusions: Most EVE-associated AEs occur soon after initiation of therapy, are typically of mild or moderate severity, and are generally manageable with dose reduction and interruption. Discontinuation due to toxicity was uncommon. Understanding the time course of class-effect AEs will help inform preventive and monitoring strategies as well as patient education.
Trial registration number: NCT00863655.
Keywords: advanced breast cancer; everolimus; mammalian target of rapamycin (mTOR); safety.
Figures

Comment in
-
Adverse events with everolimus in BOLERO-2.Ann Oncol. 2014 Sep;25(9):1861. doi: 10.1093/annonc/mdu195. Epub 2014 May 29. Ann Oncol. 2014. PMID: 24875799 No abstract available.
-
Reply to the letter to the editor 'Everolimus, when combined with exemestane, adds toxicity with minimal benefit for women with breast cancer' Tannock and Pond.Ann Oncol. 2014 Oct;25(10):2096-2098. doi: 10.1093/annonc/mdu373. Epub 2014 Aug 1. Ann Oncol. 2014. PMID: 25085504 No abstract available.
-
Everolimus, when combined with exemestane, adds toxicity with minimal benefit for women with breast cancer.Ann Oncol. 2014 Oct;25(10):2096. doi: 10.1093/annonc/mdu371. Epub 2014 Aug 1. Ann Oncol. 2014. PMID: 25085505 No abstract available.
References
-
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Breast Cancer. , Version 2.2013 www.nccn.com. (5 July 2013, date last accessed) - PubMed
-
- Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1) Breast. 2012;21:242–252. - PubMed
-
- Beeram M, Tan QT, Tekmal RR, et al. Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann Oncol. 2007;18:1323–1328. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

