Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia
- PMID: 24615777
- PMCID: PMC4123414
- DOI: 10.1182/blood-2013-11-535047
Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia
Abstract
In a phase 1 trial, idelalisib (GS-1101, CAL-101), a selective inhibitor of the lipid kinase PI3Kδ, was evaluated in 54 patients with relapsed/refractory chronic lymphocytic leukemia (CLL) with adverse characteristics including bulky lymphadenopathy (80%), extensive prior therapy (median 5 [range 2-14] prior regimens), treatment-refractory disease (70%), unmutated IGHV (91%), and del17p and/or TP53 mutations (24%). Patients were treated at 6 dose levels of oral idelalisib (range 50-350 mg once or twice daily) and remained on continuous therapy while deriving clinical benefit. Idelalisib-mediated inhibition of PI3Kδ led to abrogation of Akt phosphorylation in patient CLL cells and significantly reduced serum levels of CLL-related chemokines. The most commonly observed grade ≥3 adverse events were pneumonia (20%), neutropenic fever (11%), and diarrhea (6%). Idelalisib treatment resulted in nodal responses in 81% of patients. The overall response rate was 72%, with 39% of patients meeting the criteria for partial response per IWCLL 2008 and 33% meeting the recently updated criteria of PR with treatment-induced lymphocytosis.(1,2) The median progression-free survival for all patients was 15.8 months. This study demonstrates the clinical utility of inhibiting the PI3Kδ pathway with idelalisib. Our findings support the further development of idelalisib in patients with CLL. These trials were registered at clinicaltrials.gov as #NCT00710528 and #NCT01090414.
© 2014 by The American Society of Hematology.
Figures
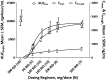


 ).
).  denotes nonevaluable patients (without a follow-up on-treatment tumor assessment) and
denotes nonevaluable patients (without a follow-up on-treatment tumor assessment) and  denotes the presence of a del17p or TP53 mutation. Forty-one patients were assessed by computed tomography scan and 9 by physical examination. Four patients did not have a follow-up assessment. (B) Rate of best on-treatment lymph node response (≥50% reduction in SPD of measured lymph nodes) and best on-treatment change from baseline in SPD of measured lymph nodes by dosing regimen (N = 50). (C) Mean changes in the SPD of measured lymph nodes and in the ALC by time during the primary study. (D) Hb concentration, platelet counts, and ANC by time during the primary study; includes only patients with baseline anemia (Hb <110 g/L), thrombocytopenia (platelets <100 × 109/L), or neutropenia (ANC <1.5 × 109/L). ALC, absolute lymphocyte count; LNRR, lymph node response rate.
denotes the presence of a del17p or TP53 mutation. Forty-one patients were assessed by computed tomography scan and 9 by physical examination. Four patients did not have a follow-up assessment. (B) Rate of best on-treatment lymph node response (≥50% reduction in SPD of measured lymph nodes) and best on-treatment change from baseline in SPD of measured lymph nodes by dosing regimen (N = 50). (C) Mean changes in the SPD of measured lymph nodes and in the ALC by time during the primary study. (D) Hb concentration, platelet counts, and ANC by time during the primary study; includes only patients with baseline anemia (Hb <110 g/L), thrombocytopenia (platelets <100 × 109/L), or neutropenia (ANC <1.5 × 109/L). ALC, absolute lymphocyte count; LNRR, lymph node response rate.
References
-
- Hallek M, Cheson BD, Catovsky D, et al. Response assessment in chronic lymphocytic leukemia treated with novel agents causing an increase of peripheral blood lymphocytes [letter]. Blood. 2012;119(23):5348.
-
- Dighiero G, Hamblin TJ. Chronic lymphocytic leukaemia. Lancet. 2008;371(9617):1017–1029. - PubMed
-
- Hallek M, Fischer K, Fingerle-Rowson G, et al. International Group of Investigators; German Chronic Lymphocytic Leukaemia Study Group. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

