Cardiolipin prevents membrane translocation and permeabilization by daptomycin
- PMID: 24616102
- PMCID: PMC4002069
- DOI: 10.1074/jbc.M114.554444
Cardiolipin prevents membrane translocation and permeabilization by daptomycin
Abstract
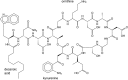
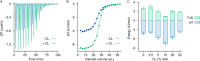
Daptomycin is an acidic lipopeptide antibiotic that, in the presence of calcium, forms oligomeric pores on membranes containing phosphatidylglycerol. It is clinically used against various Gram-positive bacteria such as Staphylococcus aureus and Enterococcus species. Genetic studies have indicated that an increased content of cardiolipin in the bacterial membrane may contribute to bacterial resistance against the drug. Here, we used a liposome model to demonstrate that cardiolipin directly inhibits membrane permeabilization by daptomycin. When cardiolipin is added at molar fractions of 10 or 20% to membranes containing phosphatidylglycerol, daptomycin no longer forms pores or translocates to the inner membrane leaflet. Under the same conditions, daptomycin continues to form oligomers; however, these oligomers contain only close to four subunits, which is approximately half as many as observed on membranes without cardiolipin. The collective findings lead us to propose that a daptomycin pore consists of two aligned tetramers in opposite leaflets and that cardiolipin prevents the translocation of tetramers to the inner leaflet, thereby forestalling the formation of complete, octameric pores. Our findings suggest a possible mechanism by which cardiolipin may mediate resistance to daptomycin, and they provide new insights into the action mode of this important antibiotic.
Keywords: Antibiotic Resistance; Antibiotics Action; Cardiolipin; Fluorescence; Isothermal Titration Calorimetry; Langmuir Monolayers; Lipopeptides; Liposomes; Phosphatidylglycerol.
Figures






References
-
- Baltz R. H. (2009) Daptomycin: mechanisms of action and resistance, and biosynthetic engineering. Curr. Opin. Chem. Biol. 13, 144–151 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

