O-GlcNAc transferase is critical for transducin-like enhancer of split (TLE)-mediated repression of canonical Wnt signaling
- PMID: 24616106
- PMCID: PMC4002120
- DOI: 10.1074/jbc.M114.553859
O-GlcNAc transferase is critical for transducin-like enhancer of split (TLE)-mediated repression of canonical Wnt signaling
Abstract
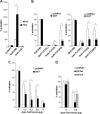
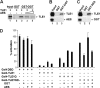
The Drosophila Groucho protein and its mammalian orthologues the transducin-like enhancers of split (TLEs) are critical transcriptional corepressors that repress Wnt and other signaling pathways. Although it is known that Groucho/TLEs are recruited to target genes by pathway-specific transcription factors, molecular events after the corepressor recruitment are largely unclear. We report that association of TLEs with O-GlcNAc transferase, an enzyme that catalyzes posttranslational modification of proteins by O-linked N-acetylglucosamine, is essential for TLE-mediated transcriptional repression. Removal of O-GlcNAc from Wnt-responsive gene promoters is critical for gene activation from Wnt-responsive promoters. Thus, these studies identify a molecular mechanism by which Groucho/TLEs repress gene transcription and provide a model whereby O-GlcNAc may control distinct intracellular signaling pathways.
Keywords: Gene Silencing; Groucho/TLE; O-GlcNAc; OGT; Signal Transduction; Transcription Repressor; Wnt Pathway.
Figures



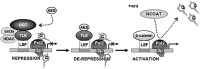
Similar articles
-
Groucho binds two conserved regions of LEF-1 for HDAC-dependent repression.BMC Cancer. 2009 May 21;9:159. doi: 10.1186/1471-2407-9-159. BMC Cancer. 2009. PMID: 19460168 Free PMC article.
-
Roles of transducin-like enhancer of split (TLE) family proteins in tumorigenesis and immune regulation.Front Cell Dev Biol. 2022 Nov 11;10:1010639. doi: 10.3389/fcell.2022.1010639. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36438567 Free PMC article. Review.
-
Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: coupling protein O-GlcNAcylation to transcriptional repression.Cell. 2002 Jul 12;110(1):69-80. doi: 10.1016/s0092-8674(02)00810-3. Cell. 2002. PMID: 12150998
-
The Groucho/transducin-like enhancer of split transcriptional repressors interact with the genetically defined amino-terminal silencing domain of histone H3.J Biol Chem. 1997 Oct 17;272(42):26604-10. doi: 10.1074/jbc.272.42.26604. J Biol Chem. 1997. PMID: 9334241
-
The Groucho/Transducin-like enhancer of split protein family in animal development.IUBMB Life. 2015 Jul;67(7):472-81. doi: 10.1002/iub.1395. Epub 2015 Jul 14. IUBMB Life. 2015. PMID: 26172616 Free PMC article. Review.
Cited by
-
Differentiation of imatinib -resistant chronic myeloid leukemia cells with BCR-ABL-T315I mutation induced by Jiyuan Oridonin A.J Cancer. 2023 May 5;14(7):1182-1194. doi: 10.7150/jca.83219. eCollection 2023. J Cancer. 2023. PMID: 37215441 Free PMC article.
-
Mapping the signaling network of BIN2 kinase using TurboID-mediated biotin labeling and phosphoproteomics.Plant Cell. 2023 Mar 15;35(3):975-993. doi: 10.1093/plcell/koad013. Plant Cell. 2023. PMID: 36660928 Free PMC article.
-
The prognostic role of the Transducin-like Enhancer of split protein family in lung adenocarcinoma.Transl Lung Cancer Res. 2021 Jul;10(7):3251-3263. doi: 10.21037/tlcr-21-582. Transl Lung Cancer Res. 2021. PMID: 34430362 Free PMC article.
-
Inhibition of O-GlcNAc transferase activates type I interferon-dependent antitumor immunity by bridging cGAS-STING pathway.bioRxiv [Preprint]. 2024 Jun 11:2023.12.14.571787. doi: 10.1101/2023.12.14.571787. bioRxiv. 2024. Update in: Elife. 2024 Oct 04;13:RP94849. doi: 10.7554/eLife.94849. PMID: 38168435 Free PMC article. Updated. Preprint.
-
Human disease modeling reveals integrated transcriptional and epigenetic mechanisms of NOTCH1 haploinsufficiency.Cell. 2015 Mar 12;160(6):1072-86. doi: 10.1016/j.cell.2015.02.035. Cell. 2015. PMID: 25768904 Free PMC article.
References
-
- Dehni G., Liu Y., Husain J., Stifani S. (1995) TLE expression correlates with mouse embryonic segmentation, neurogenesis, and epithelial determination. Mech. Dev. 53, 369–381 - PubMed
-
- Li S. S. (2000) Structure and function of the Groucho gene family and encoded transcriptional corepressor proteins from human, mouse, rat, Xenopus, Drosophila and nematode. Proc. Natl. Sci. Counc. Repub. China B 24, 47–55 - PubMed
-
- Grbavec D., Lo R., Liu Y., Stifani S. (1998) Transducin-like Enhancer of split 2, a mammalian homologue of Drosophila Groucho, acts as a transcriptional repressor, interacts with Hairy/Enhancer of split proteins, and is expressed during neuronal development. Eur. J. Biochem. 258, 339–349 - PubMed
-
- Gasperowicz M., Otto F. (2005) Mammalian Groucho homologs: redundancy or specificity? J. Cell. Biochem. 95, 670–687 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

