Bleb-driven chemotaxis of Dictyostelium cells
- PMID: 24616222
- PMCID: PMC3998804
- DOI: 10.1083/jcb.201306147
Bleb-driven chemotaxis of Dictyostelium cells
Abstract
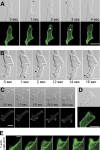
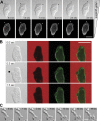
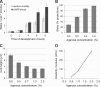
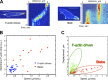
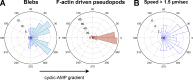
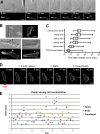
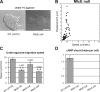
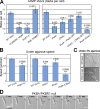
Blebs and F-actin-driven pseudopods are alternative ways of extending the leading edge of migrating cells. We show that Dictyostelium cells switch from using predominantly pseudopods to blebs when migrating under agarose overlays of increasing stiffness. Blebs expand faster than pseudopods leaving behind F-actin scars, but are less persistent. Blebbing cells are strongly chemotactic to cyclic-AMP, producing nearly all of their blebs up-gradient. When cells re-orientate to a needle releasing cyclic-AMP, they stereotypically produce first microspikes, then blebs and pseudopods only later. Genetically, blebbing requires myosin-II and increases when actin polymerization or cortical function is impaired. Cyclic-AMP induces transient blebbing independently of much of the known chemotactic signal transduction machinery, but involving PI3-kinase and downstream PH domain proteins, CRAC and PhdA. Impairment of this PI3-kinase pathway results in slow movement under agarose and cells that produce few blebs, though actin polymerization appears unaffected. We propose that mechanical resistance induces bleb-driven movement in Dictyostelium, which is chemotactic and controlled through PI3-kinase.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

