Improving the care of children with advanced cancer by using an electronic patient-reported feedback intervention: results from the PediQUEST randomized controlled trial
- PMID: 24616307
- PMCID: PMC3970170
- DOI: 10.1200/JCO.2013.51.5981
Improving the care of children with advanced cancer by using an electronic patient-reported feedback intervention: results from the PediQUEST randomized controlled trial
Abstract
Purpose: This study aimed to determine whether feeding back patient-reported outcomes (PROs) to providers and families of children with advanced cancer improves symptom distress and health-related quality of life (HRQoL).
Patients and methods: This study was a parallel, multicentered pilot randomized controlled trial. At most once per week, children age ≥ 2 years old with advanced cancer or their parent completed the computer-based Pediatric Quality of Life and Evaluation of Symptoms Technology (PediQUEST) survey consisting of age- and respondent-adapted versions of the Memorial Symptom Assessment Scale (MSAS), Pediatric Quality of Life Inventory 4.0 Generic Core Scales (PedsQL4.0), and an overall Sickness question. In the intervention group (n = 51), oncologists and families received printed reports summarizing PROs; e-mails were sent to oncologists and subspecialists when predetermined scores were exceeded. No feedback was provided in the control group (n = 53). Primary outcomes included linear trends of MSAS, PedsQL4.0 total and subscale scores, and Sickness scores during 20 weeks of follow-up, along with child, parent, and provider satisfaction with PediQUEST feedback.
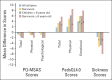
Results: Feedback did not significantly affect average MSAS, PedsQL4.0, or Sickness score trends. Post hoc subgroup analyses among children age ≥ 8 years who survived 20 weeks showed that feedback improved PedsQL4.0 emotional (+8.1; 95% CI, 1.8 to 14.4) and Sickness (-8.2; 95% CI, -14.2 to -2.2) scores. PediQUEST reports were valued by children, parents, and providers and contributed at least sometimes to physician initiation of a psychosocial consult (56%).
Conclusion: Although routine feedback of PROs did not significantly affect the child's symptoms or HRQoL, changes were in expected directions and improvements observed in emotional HRQoL through exploratory analyses were encouraging. Importantly, children, parents, and providers value PRO feedback.
Trial registration: ClinicalTrials.gov NCT01838564.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Using patient-reported outcomes in clinical practice: a promising approach?J Clin Oncol. 2014 Apr 10;32(11):1099-100. doi: 10.1200/JCO.2013.53.4271. Epub 2014 Mar 10. J Clin Oncol. 2014. PMID: 24616314 No abstract available.
References
-
- American Cancer Society. Atlanta, GA: American Cancer Society; 2012. Cancer Facts and Figures 2012.
-
- Heath JA, Clarke NE, Donath SM, et al. Symptoms and suffering at the end of life in children with cancer: An Australian perspective. Med J Aust. 2010;192:71–75. - PubMed
-
- Jalmsell L, Kreicbergs U, Onelöv E, et al. Symptoms affecting children with malignancies during the last month of life: A nationwide follow-up. Pediatrics. 2006;117:1314–1320. - PubMed
-
- Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med. 2000;342:326–333. - PubMed
-
- Tomlinson D, Hinds PS, Bartels U, et al. Parent reports of quality of life for pediatric patients with cancer with no realistic chance of cure. J Clin Oncol. 2011;29:639–645. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

