Clinical pharmacogenetics implementation: approaches, successes, and challenges
- PMID: 24616371
- PMCID: PMC4076109
- DOI: 10.1002/ajmg.c.31390
Clinical pharmacogenetics implementation: approaches, successes, and challenges
Abstract
Current challenges exist to widespread clinical implementation of genomic medicine and pharmacogenetics. The University of Florida (UF) Health Personalized Medicine Program (PMP) is a pharmacist-led, multidisciplinary initiative created in 2011 within the UF Clinical Translational Science Institute. Initial efforts focused on pharmacogenetics, with long-term goals to include expansion to disease-risk prediction and disease stratification. Herein we describe the processes for development of the program, the challenges that were encountered and the clinical acceptance by clinicians of the genomic medicine implementation. The initial clinical implementation of the UF PMP began in June 2012 and targeted clopidogrel use and the CYP2C19 genotype in patients undergoing left heart catheterization and percutaneous-coronary intervention (PCI). After 1 year, 1,097 patients undergoing left heart catheterization were genotyped preemptively, and 291 of those underwent subsequent PCI. Genotype results were reported to the medical record for 100% of genotyped patients. Eighty patients who underwent PCI had an actionable genotype, with drug therapy changes implemented in 56 individuals. Average turnaround time from blood draw to genotype result entry in the medical record was 3.5 business days. Seven different third party payors, including Medicare, reimbursed for the test during the first month of billing, with an 85% reimbursement rate for outpatient claims that were submitted in the first month. These data highlight multiple levels of success in clinical implementation of genomic medicine.
Keywords: CYP2C19; genomic medicine; implementation; personalized medicine; pharmacogenetics.
© 2014 Wiley Periodicals, Inc.
Figures
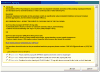

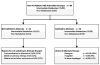
References
-
- Hamilton AB, Oishi S, Yano EM, Gammage CE, Marshall NJ, Scheuner MT. Factors influencing organizational adoption and implementation of clinical genetic services. Genet Med. 2014;16:238–245. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

