Crystal structure of human soluble adenylate cyclase reveals a distinct, highly flexible allosteric bicarbonate binding pocket
- PMID: 24616449
- PMCID: PMC4506562
- DOI: 10.1002/cmdc.201300480
Crystal structure of human soluble adenylate cyclase reveals a distinct, highly flexible allosteric bicarbonate binding pocket
Abstract
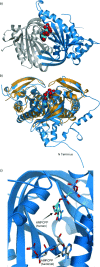
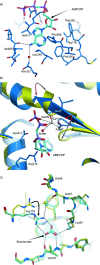
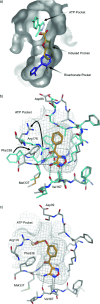
Soluble adenylate cyclases catalyse the synthesis of the second messenger cAMP through the cyclisation of ATP and are the only known enzymes to be directly activated by bicarbonate. Here, we report the first crystal structure of the human enzyme that reveals a pseudosymmetrical arrangement of two catalytic domains to produce a single competent active site and a novel discrete bicarbonate binding pocket. Crystal structures of the apo protein, the protein in complex with α,β-methylene adenosine 5'-triphosphate (AMPCPP) and calcium, with the allosteric activator bicarbonate, and also with a number of inhibitors identified using fragment screening, all show a flexible active site that undergoes significant conformational changes on binding of ligands. The resulting nanomolar-potent inhibitors that were developed bind at both the substrate binding pocket and the allosteric site, and can be used as chemical probes to further elucidate the function of this protein.
Keywords: allosterism; drug discovery; enzyme regulation; fragment screening; structural biology.
© 2014 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Figures
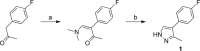

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

