Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells
- PMID: 24616478
- PMCID: PMC3984038
- DOI: 10.4049/jimmunol.1302062
Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells
Abstract
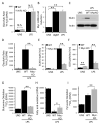
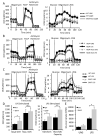
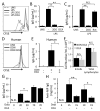
B cell activation leads to proliferation and Ab production that can protect from pathogens or promote autoimmunity. Regulation of cell metabolism is essential to support the demands of lymphocyte growth and effector function and may regulate tolerance. In this study, we tested the regulation and role of glucose uptake and metabolism in the proliferation and Ab production of control, anergic, and autoimmune-prone B cells. Control B cells had a balanced increase in lactate production and oxygen consumption following activation, with proportionally increased glucose transporter Glut1 expression and mitochondrial mass upon either LPS or BCR stimulation. This contrasted with metabolic reprogramming of T cells, which had lower glycolytic flux when resting but disproportionately increased this pathway upon activation. Importantly, tolerance greatly affected B cell metabolic reprogramming. Anergic B cells remained metabolically quiescent, with only a modest increase in glycolysis and oxygen consumption with LPS stimulation. B cells chronically stimulated with elevated BAFF, however, rapidly increased glycolysis and Ab production upon stimulation. Induction of glycolysis was critical for Ab production, as glycolytic inhibition with the pyruvate dehydrogenase kinase inhibitor dichloroacetate sharply suppressed B cell proliferation and Ab secretion in vitro and in vivo. Furthermore, B cell-specific deletion of Glut1 led to reduced B cell numbers and impaired Ab production in vivo. Together, these data show that activated B cells require Glut1-dependent metabolic reprogramming to support proliferation and Ab production that is distinct from T cells and that this glycolytic reprogramming is regulated in tolerance.
Figures





References
-
- Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56AI102074/AI/NIAID NIH HHS/United States
- R56 AI102074/AI/NIAID NIH HHS/United States
- R03 AI106835/AI/NIAID NIH HHS/United States
- R01AI81597/AI/NIAID NIH HHS/United States
- U01 HL087947/HL/NHLBI NIH HHS/United States
- P30 CA014236/CA/NCI NIH HHS/United States
- R01 GM052735/GM/NIGMS NIH HHS/United States
- R01 HL108006/HL/NHLBI NIH HHS/United States
- R01GM52735/GM/NIGMS NIH HHS/United States
- R01HL108006/HL/NHLBI NIH HHS/United States
- R01 AI047891/AI/NIAID NIH HHS/United States
- U01HL087947/HL/NHLBI NIH HHS/United States
- R01AI47891/AI/NIAID NIH HHS/United States
- R01AI56363/AI/NIAID NIH HHS/United States
- U19 AI056363/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

