Neural pathways that control the glucose counterregulatory response
- PMID: 24616659
- PMCID: PMC3935387
- DOI: 10.3389/fnins.2014.00038
Neural pathways that control the glucose counterregulatory response
Abstract
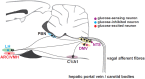
Glucose is an essential metabolic substrate for all bodily tissues. The brain depends particularly on a constant supply of glucose to satisfy its energy demands. Fortunately, a complex physiological system has evolved to keep blood glucose at a constant level. The consequences of poor glucose homeostasis are well-known: hyperglycemia associated with uncontrolled diabetes can lead to cardiovascular disease, neuropathy and nephropathy, while hypoglycemia can lead to convulsions, loss of consciousness, coma, and even death. The glucose counterregulatory response involves detection of declining plasma glucose levels and secretion of several hormones including glucagon, adrenaline, cortisol, and growth hormone (GH) to orchestrate the recovery from hypoglycemia. Low blood glucose leads to a low brain glucose level that is detected by glucose-sensing neurons located in several brain regions such as the ventromedial hypothalamus, the perifornical region of the lateral hypothalamus, the arcuate nucleus (ARC), and in several hindbrain regions. This review will describe the importance of the glucose counterregulatory system and what is known of the neurocircuitry that underpins it.
Keywords: adrenaline; counterregulation; glucagon; glucose sensing; hypoglycemia; perifornical hypothalamus; rostral ventrolateral medulla; ventromedial hypothalamus.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

