Integration and regulation of glomerular inhibition in the cerebellar granular layer circuit
- PMID: 24616663
- PMCID: PMC3933946
- DOI: 10.3389/fncel.2014.00055
Integration and regulation of glomerular inhibition in the cerebellar granular layer circuit
Erratum in
- Front Cell Neurosci. 2014 Oct;8:342
-
Corrigendum: Integration and regulation of glomerular inhibition in the cerebellar granular layer circuit.Front Cell Neurosci. 2016 Feb 16;10:31. doi: 10.3389/fncel.2016.00031. eCollection 2016. Front Cell Neurosci. 2016. PMID: 26909024 Free PMC article.
Abstract
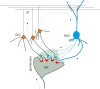
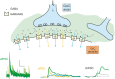
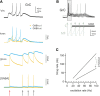
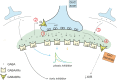
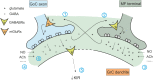
Inhibitory synapses can be organized in different ways and be regulated by a multitude of mechanisms. One of the best known examples is provided by the inhibitory synapses formed by Golgi cells onto granule cells in the cerebellar glomeruli. These synapses are GABAergic and inhibit granule cells through two main mechanisms, phasic and tonic. The former is based on vesicular neurotransmitter release, the latter on the establishment of tonic γ-aminobutyric acid (GABA) levels determined by spillover and regulation of GABA uptake. The mechanisms of post-synaptic integration have been clarified to a considerable extent and have been shown to differentially involve α1 and α6 subunit-containing GABA-A receptors. Here, after reviewing the basic mechanisms of GABAergic transmission in the cerebellar glomeruli, we examine how inhibition controls signal transfer at the mossy fiber-granule cell relay. First of all, we consider how vesicular release impacts on signal timing and how tonic GABA levels control neurotransmission gain. Then, we analyze the integration of these inhibitory mechanisms within the granular layer network. Interestingly, it turns out that glomerular inhibition is just one element in a large integrated signaling system controlled at various levels by metabotropic receptors. GABA-B receptor activation by ambient GABA regulates glutamate release from mossy fibers through a pre-synaptic cross-talk mechanisms, GABA release through pre-synaptic auto-receptors, and granule cell input resistance through post-synaptic receptor activation and inhibition of a K inward-rectifier current. Metabotropic glutamate receptors (mGluRs) control GABA release from Golgi cell terminals and Golgi cell input resistance and autorhythmic firing. This complex set of mechanisms implements both homeostatic and winner-take-all processes, providing the basis for fine-tuning inhibitory neurotransmission and for optimizing signal transfer through the cerebellar cortex.
Keywords: GABA receptors; Golgi cells; cerebellum; granule cells; synaptic inhibition.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

