The role of iron in anthracycline cardiotoxicity
- PMID: 24616701
- PMCID: PMC3935484
- DOI: 10.3389/fphar.2014.00025
The role of iron in anthracycline cardiotoxicity
Abstract
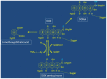
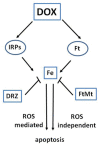
The clinical use of the antitumor anthracycline Doxorubicin is limited by the risk of severe cardiotoxicity. The mechanisms underlying anthracycline-dependent cardiotoxicity are multiple and remain uncompletely understood, but many observations indicate that interactions with cellular iron metabolism are important. Convincing evidence showing that iron plays a role in Doxorubicin cardiotoxicity is provided by the protecting efficacy of iron chelation in patients and experimental models, and studies showing that iron overload exacerbates the cardiotoxic effects of the drug, but the underlying molecular mechanisms remain to be completely characterized. Since anthracyclines generate reactive oxygen species, increased iron-catalyzed formation of free radicals appears an obvious explanation for the aggravating role of iron in Doxorubicin cardiotoxicity, but antioxidants did not offer protection in clinical settings. Moreover, how the interaction between reactive oxygen species and iron damages heart cells exposed to Doxorubicin is still unclear. This review discusses the pathogenic role of the disruption of iron homeostasis in Doxorubicin-mediated cardiotoxicity in the context of current and future pharmacologic approaches to cardioprotection.
Keywords: anthracyclines; doxorubicin; heart; iron; reactive oxygen species.
Figures
References
-
- Asensio-López M. C., Sanchez-Mas J., Pascual-Figal D. A., De Torre C., Valdes M., Lax A. (2013a). Ferritin heavy chain as main mediator of preventive effect of metformin against mitochondrial damage induced by doxorubicin in cardiomyocytes. Free Radic. Biol. Med. 67C 19–29 10.1016/j.freeradbiomed.2013.11.003 - DOI - PubMed
-
- Asensio-López M. C., Sánchez-Más J., Pascual-Figal D. A., Abenza S., Pérez-Martïnez M. T., Valdés M., et al. (2013b). Involvement of ferritin heavy chain in the preventive effect of metformin against doxorubicin-induced cardiotoxicity. Free Radic. Biol. Med. 57 188–200 10.1016/j.freeradbiomed.2012.09.009 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

