Thyroid hormone and seasonal rhythmicity
- PMID: 24616714
- PMCID: PMC3935485
- DOI: 10.3389/fendo.2014.00019
Thyroid hormone and seasonal rhythmicity
Abstract
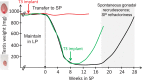
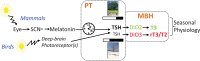
Living organisms show seasonality in a wide array of functions such as reproduction, fattening, hibernation, and migration. At temperate latitudes, changes in photoperiod maintain the alignment of annual rhythms with predictable changes in the environment. The appropriate physiological response to changing photoperiod in mammals requires retinal detection of light and pineal secretion of melatonin, but extraretinal detection of light occurs in birds. A common mechanism across all vertebrates is that these photoperiod-regulated systems alter hypothalamic thyroid hormone (TH) conversion. Here, we review the evidence that a circadian clock within the pars tuberalis of the adenohypophysis links photoperiod decoding to local changes of TH signaling within the medio-basal hypothalamus (MBH) through a conserved thyrotropin/deiodinase axis. We also focus on recent findings which indicate that, beyond the photoperiodic control of its conversion, TH might also be involved in longer-term timing processes of seasonal programs. Finally, we examine the potential implication of kisspeptin and RFRP3, two RF-amide peptides expressed within the MBH, in seasonal rhythmicity.
Keywords: GnRH neurons; RF-amide; kisspeptins; melatonin rhythm; pars tuberalis; reproduction; seasonality.
Figures



References
-
- Lincoln GA, Short RV. Seasonal breeding: nature’s contraceptive. Recent Prog Horm Res (1980) 36:1–52 - PubMed
-
- Karsch FJ, Bittman EL, Foster DL, Goodman RL, Legan SJ, Robinson JE. Neuroendocrine basis of seasonal reproduction. Recent Prog Horm Res (1984) 40:185–232 - PubMed
-
- Zucker I. Circannual rhythms. In: Takahashi JS, Turek FW, Moore RY, editors. Handbook of Behavioural Neurobiology, Circadian Clocks. (Vol. 12), New York: Kluwer/Plenum; (2001). p. 511–28
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

