Characterization of serine hydroxymethyltransferase GlyA as a potential source of D-alanine in Chlamydia pneumoniae
- PMID: 24616885
- PMCID: PMC3935232
- DOI: 10.3389/fcimb.2014.00019
Characterization of serine hydroxymethyltransferase GlyA as a potential source of D-alanine in Chlamydia pneumoniae
Abstract
For intracellular Chlamydiaceae, there is no need to withstand osmotic challenges, and a functional cell wall has not been detected in these pathogens so far. Nevertheless, penicillin inhibits cell division in Chlamydiaceae resulting in enlarged aberrant bodies, a phenomenon known as chlamydial anomaly. D-alanine is a unique and essential component in the biosynthesis of bacterial cell walls. In free-living bacteria like Escherichia coli, penicillin-binding proteins such as monofunctional transpeptidases PBP2 and PBP3, the putative targets of penicillin in Chlamydiaceae, cross-link adjacent peptidoglycan strands via meso-diaminopimelic acid and D-Ala-D-Ala moieties of pentapeptide side chains. In the absence of genes coding for alanine racemase Alr and DadX homologs, the source of D-Ala and thus the presence of substrates for PBP2 and PBP3 activity in Chlamydiaceae has puzzled researchers for years. Interestingly, Chlamydiaceae genomes encode GlyA, a serine hydroxymethyltransferase that has been shown to exhibit slow racemization of D- and L-alanine as a side reaction in E. coli. We show that GlyA from Chlamydia pneumoniae can serve as a source of D-Ala. GlyA partially reversed the D-Ala auxotrophic phenotype of an E. coli racemase double mutant. Moreover, purified chlamydial GlyA had racemase activity on L-Ala in vitro and was inhibited by D-cycloserine, identifying GlyA, besides D-Ala ligase MurC/Ddl, as an additional target of this competitive inhibitor in Chlamydiaceae. Proof of D-Ala biosynthesis in Chlamydiaceae helps to clarify the structure of cell wall precursor lipid II and the role of chlamydial penicillin-binding proteins in the development of non-dividing aberrant chlamydial bodies and persistence in the presence of penicillin.
Keywords: D-alanine; D-cycloserine; GlyA; aberrant bodies; alanine racemase; chlamydial anomaly; penicillin; persistence.
Figures

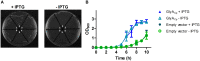
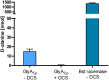
References
-
- Centers for Disease Control Prevention (CDC) (2010). Sexually transmitted diseases treatment guidelines. MMWR Recomm. Rep. 59, 1–110 - PubMed
-
- Contestabile R., Paiardini A., Pascarella S., Di Salvo M. L., D'aguanno S., Bossa F. (2001). l-Threonine aldolase, serine hydroxymethyltransferase and fungal alanine racemase. A subgroup of strictly related enzymes specialized for different functions. Eur. J. Biochem. 268, 6508–6525 10.1046/j.0014-2956.2001.02606.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

