The PAS domain-containing histidine kinase RpfS is a second sensor for the diffusible signal factor of Xanthomonas campestris
- PMID: 24617591
- PMCID: PMC4159695
- DOI: 10.1111/mmi.12577
The PAS domain-containing histidine kinase RpfS is a second sensor for the diffusible signal factor of Xanthomonas campestris
Abstract
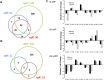
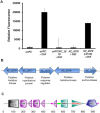
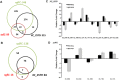
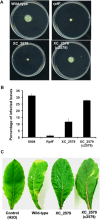
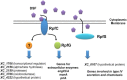
A cell-cell signalling system mediated by the fatty acid signal DSF controls the virulence of Xanthomonas campestris pv. campestris (Xcc) to plants. The synthesis and recognition of the DSF signal depends upon different Rpf proteins. DSF signal generation requires RpfF whereas signal perception and transduction depends upon the sensor RpfC and regulator RpfG. Detailed analyses of the regulatory roles of different Rpf proteins have suggested the occurrence of further sensors for DSF. Here we have used a mutagenesis approach coupled with high-resolution transcriptional analysis to identify XC_2579 (RpfS) as a second sensor for DSF in Xcc. RpfS is a complex sensor kinase predicted to have multiple Per/Arnt/Sim (PAS) domains, a histidine kinase domain and a C-terminal receiver (REC) domain. Isothermal calorimetry showed that DSF bound to the isolated N-terminal PAS domain with a Kd of 1.4 μM. RpfS controlled expression of a sub-set of genes distinct from those controlled by RpfC to include genes involved in type IV secretion and chemotaxis. Mutation of XC_2579 was associated with a reduction in virulence of Xcc to Chinese Radish when assayed by leaf spraying but not by leaf inoculation, suggesting a role for RpfS-controlled factors in the epiphytic phase of the disease cycle.
© 2014 The Authors. Molecular Microbiology published by John Wiley & Sons Ltd.
Figures

References
-
- An, S.Q., Febrer, M., McCarthy, Y., Tang, D.J., Clissold, L., Kaithakotti, G., et al (2013) High‐resolution transcriptional analysis of the regulatory influence of cell‐to‐cell signalling reveals novel genes that contribute to Xanthomonas phytopathogenesis. Mol Microbiol 88: 1058–1069 - PMC - PubMed
-
- Dow, M. (2008) Diversification of the function of cell‐to‐cell signaling in regulation of virulence within plant pathogenic xanthomonads. Sci Signal 1: pe23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

